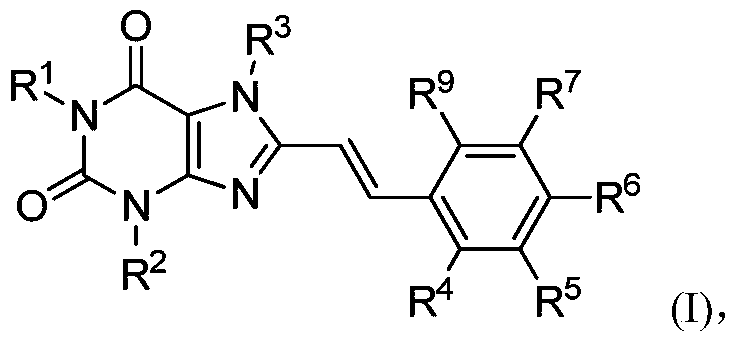
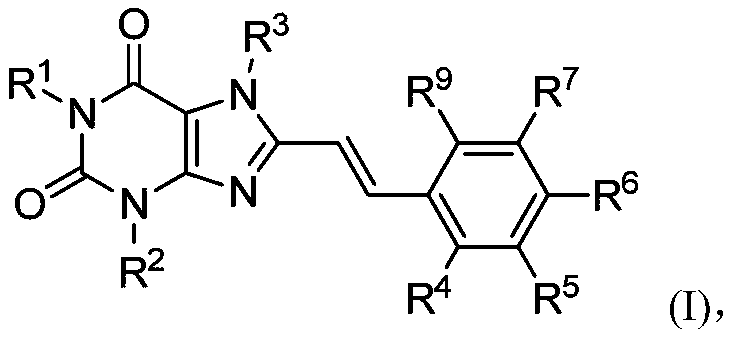
8-substituted styryl xanthine derivative and application thereof
An alkyl and aryl technology, applied in the field of medicine, can solve problems such as the distribution limitation of adenosine A, and achieve the effects of good brain/plasma ratio, good metabolic stability, and good pharmacokinetic properties
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
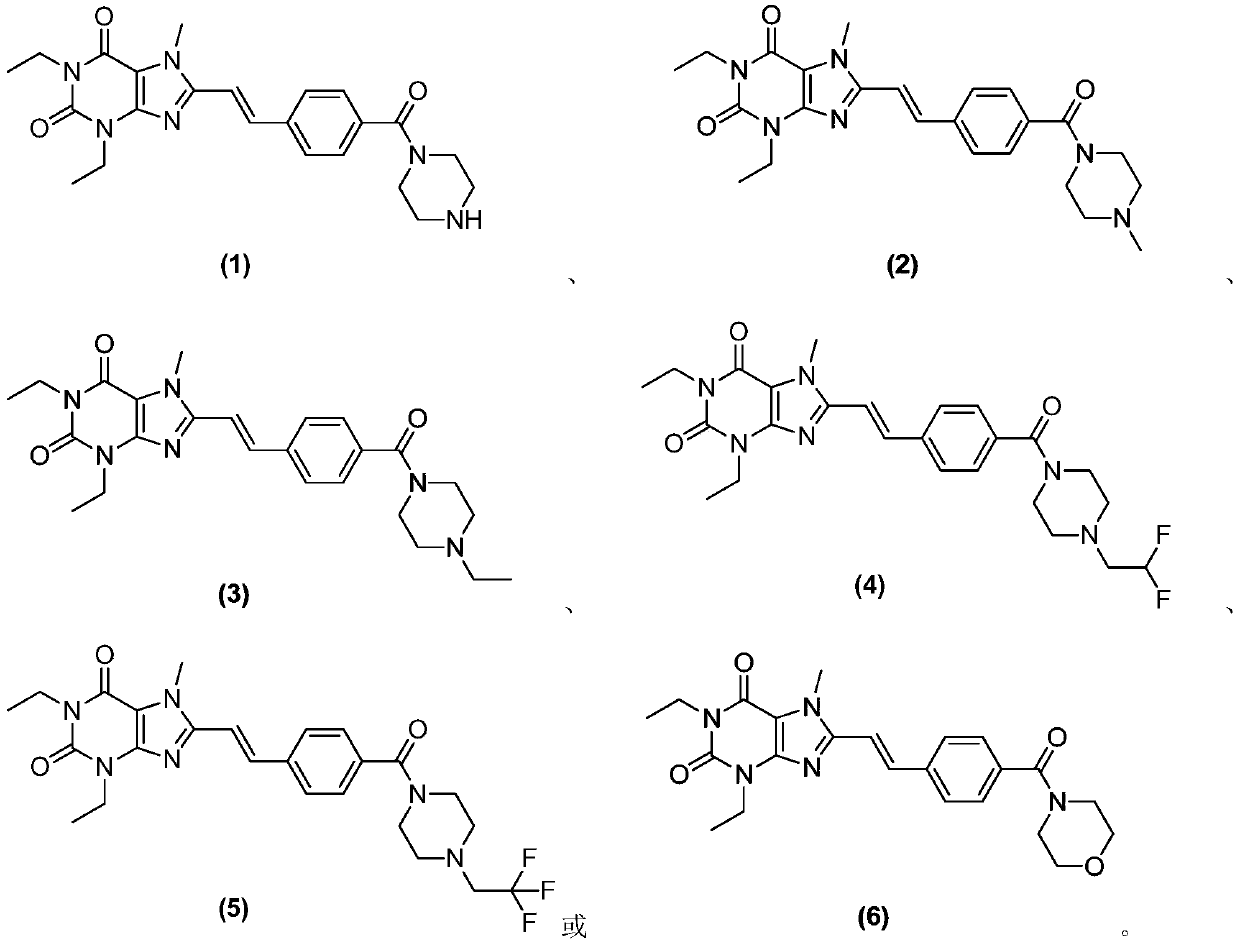
[0266] Example 1 (E)-1,3-diethyl-7-methyl-8-(4-(piperazine-1-carbonyl)styryl)-1H-purine-2,6(3H,7H) - Synthesis of diketones
[0267]
[0268] Step 1) Synthesis of (E)-3-(4-(methoxycarbonyl)phenyl)acrylic acid
[0269]
[0270] Methyl 4-formylbenzoate (6.0 g, 36.5 mmol), malonic acid (5.7 g, 54.8 mmol), piperidine (2.2 mL, 24 mmol) and pyridine (10 mL) were added to a 100 mL single-necked round bottom flask, The oil bath was reacted at 100°C for 2 hours. Cool to room temperature after the reaction, spin dry under reduced pressure, add hydrochloric acid (12M) to adjust pH = 2, filter, and dry at 60°C to obtain a white solid (7.3g, 96.9%).
[0271] MS(ESI,pos.ion)m / z:207.1[M+H] + ;
[0272] 1 H NMR (400MHz, DMSO-d 6 )δ (ppm) 7.97 (d, J = 8.2Hz, 2H), 7.82 (d, J = 8.2Hz, 2H), 7.63 (d, J = 16.0Hz, 1H), 6.66 (d, J = 16.0Hz, 1H), 3.86(s, 3H).
[0273] Step 2) (E)-methyl 4-(3-((6-amino-1,3-diethyl-2,4-dioxo-1,2,3,4-tetrahydropyrimidine-5- Synthesis of (yl)amino)-3-o...
Embodiment 2
[0298] Example 2 (E)-1,3-diethyl-7-methyl-8-(4-(4-methylpiperazine-1-carbonyl)styryl)-1H-purine-2,6( Synthesis of 3H,7H)-diketones
[0299]
[0300] (E)-1,3-diethyl-7-methyl-8-(4-(piperazine-1-carbonyl)styryl)-1H-purine-2,6(3H, 7H)-diketone (0.6g, 1.37mmol) and methanol (10mL) were added to a 100mL single-necked round bottom flask, and aqueous formaldehyde (1.02mL, 13.8mmol, 37mass%) and acetic acid (0.24mL, 4.13mmol) were added, stirred After reacting for 15 minutes, add sodium cyanoborohydride (0.27g, 4.13mmol), transfer to 25°C and continue stirring for 12 hours; stop the reaction, add water (30mL), dichloromethane extraction (50mL), liquid separation, organic The phase was spin-dried under reduced pressure, separated and purified by column chromatography (dichloromethane / methanol (v / v)=15 / 1) to obtain the title compound as a pale yellow solid (0.421 g, 68.1%).
[0301] MS(ESI,pos.ion)m / z:451.2[M+H] + ;
[0302] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 7.78 (d, J = 15.7Hz,...
Embodiment 3
[0303] Example 3 (E)-1,3-diethyl-7-methyl-8-(4-(4-ethylpiperazine-1-carbonyl)styryl)-1H-purine-2,6( Synthesis of 3H,7H)-diketones
[0304]
[0305] (E)-1,3-diethyl-7-methyl-8-(4-(piperazine-1-carbonyl)styryl)-1H-purine-2,6(3H, 7H)-diketone (1.0g, 2.29mmol) and acetonitrile (20mL) were added to a 100mL single-necked round-bottomed flask, N,N-diisopropylethylamine (1.13mL, 6.78mmol) was added dropwise, and ethyl iodide was added dropwise Alkane (0.38mL, 4.76mmol), continue to stir and react for 11 hours; stop the reaction, spin dry under reduced pressure, separate and purify by column chromatography (petroleum ether / ethyl acetate (v / v)=1 / 1) to obtain the title compound as yellow Solid (0.91 g, 85%).
[0306] MS(ESI,pos.ion)m / z:465.2[M+H] + ;
[0307] 1 H NMR (400MHz, CDCl 3 )δ (ppm) 7.81 (d, J = 15.7Hz, 1H), 7.63 (d, J = 8.1Hz, 2H), 7.47 (d, J = 8.1Hz, 2H), 6.98 (d, J = 15.7Hz, 1H), 4.23(q, J=7.0Hz, 2H), 4.15–4.04(m, 5H), 3.83(brs, 2H), 3.50(brs, 2H), 2.63–2.41(m, 6H),...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com