GSK-3beta/ChE double inhibitor as well as preparation method and application thereof
A technology of inhibitors and solvates, applied in the field of medicine, can solve problems such as reduction and loss of binding effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
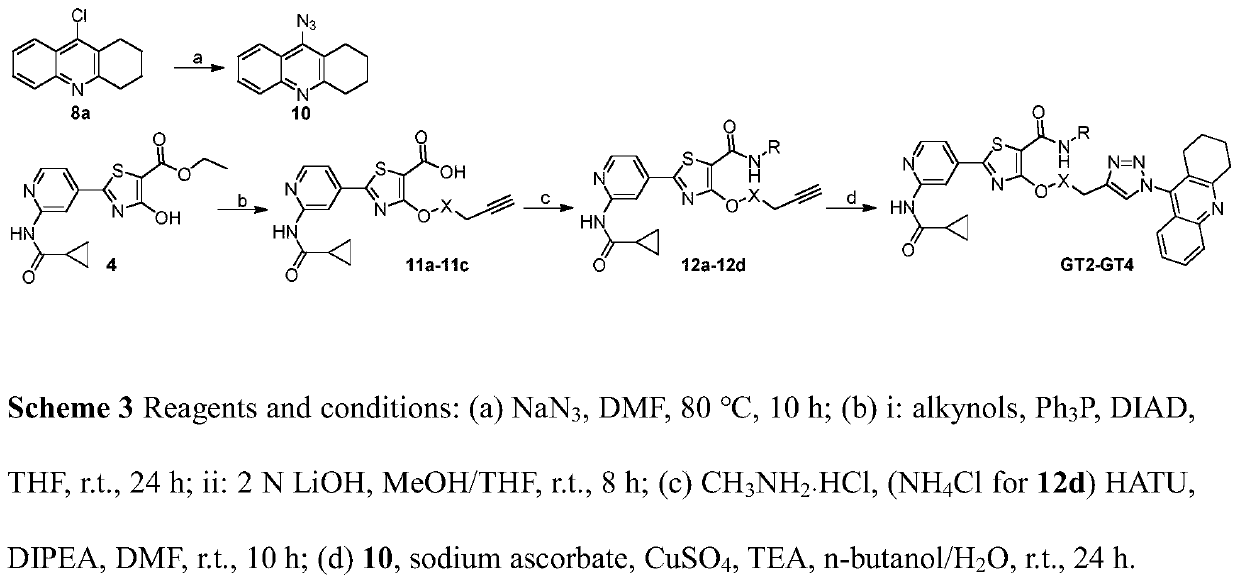
[0112] 2-(2-(Cyclopropanecarboxamido)pyridine)-4-(2-((1,2,3,4-tetrahydroacridine)amino)ethoxy)thiazole-5-carboxylic acid ethyl ester ( 2a)
[0113]
[0114] Compound 4 (666mg, 2mmol) and 9i (727mg, 3mmol) were dissolved in 55mL THF, triphenylphosphine (1.57g, 6mmol) was added, and DIAD (1.2mL, 6mmol) was slowly added dropwise under the protection of argon under ice bath conditions. ), and reacted at room temperature for 24 hours after the addition was completed and the ice bath was removed. The reaction solution was spin-dried, dissolved in DCM and separated by column chromatography to obtain the product (DCM / MeOH=30 / 1, v / v). The product was a light yellow solid with a yield of 52%. 1 H NMR (300MHz, DMSO-d 6 ,δppm): 11.04(s,1H,-CONH-),8.51(m,1H,-NHCH 2 -),8.42(m,2H,Ar-H),7.41-7.77(m,3H,Ar-H),7.41-7.49(m,2H,Ar-H),4.16(m,2H,-OCH 2 CH 3 ), 4.18(t, J=9.1Hz, 2H, -CH 2 NH-),3.29(m,2H,-CH 2 NH-),2.99(m,2H,Ar-CH 2 -),2.60(m,2H,Ar-CH 2 -),2.07(m,1H,-CHCO-),1.76(m,4H,-(CH ...
Embodiment 2
[0116] 2-(2-(Cyclopropanecarboxamido)pyridine)-4-(2-((1,2,3,4-tetrahydroacridine)amino)ethoxy)thiazole-5-carboxylic acid (2b)
[0117]
[0118] Dissolve 2a (55.7mg, 0.1mmol) in 3mL THF / MeOH mixed solution (THF / MeOH=2 / 1, v / v), add LiOH solution (400μL, 1.5mmol / mL) dropwise under ice bath, remove the ice React at room temperature for 7 hours after bathing. The completion of the reaction was detected by TLC, THF in the reaction solution was removed by rotary evaporation at low temperature, 2 times the amount of water was added, the acid was adjusted to precipitate a solid, and suction filtration was obtained to obtain a pale yellow crystalline product (60.1 mg), with a yield of 87%. 1 H NMR (300MHz, DMSO-d 6 ,δppm):10.94(s,1H,-CONH-),8.45(m,1H,-NHCH 2 -),8.39(m,1H,Ar-H),7.89(m,1H,Ar-H),7.78(m,1H,Ar-H),7.69(t,J=8.2Hz,1H,Ar-H ),7.57(m,1H,Ar-H),7.38(t,J=8.2Hz,2H,Ar-H),4.83(t,J=8.2Hz,2H,-NHCH 2 -),4.29(m,2H,-NHCH 2 -), 2.95(t, J=8.2Hz, 2H, Ar-CH 2 -), 2.66(t, J=8.2Hz, 2H, Ar...
Embodiment 3
[0120] 2-(2-(Cyclopropanecarboxamido)pyridine)-4-(2-((1,2,3,4-tetrahydroacridine)amino)ethoxy)thiazole-5-carboxamide (2c)
[0121] 2b (55.7mg, 0.1mmol) was dissolved in 8mL DMF, DIPEA (49.5μL, 0.3mmol) and HATU (53.2mg, 0.14mmol) were added, activated at room temperature for 3 hours under the protection of argon, and then NH 4 Cl (31.8 mg, 0.6 mmol) was reacted at room temperature under the protection of argon for 18 hours. The completion of the reaction was detected by TLC. The reaction solution was spin-dried, sanded, and separated by column chromatography to obtain a yellow solid product (DCM / MeOH=10 / 1, v / v), with a yield of 38%. 1 H NMR (300MHz, DMSO-d 6,δppm):11.00(s,1H,-CONH-),8.44(m,1H,-NHCH 2 -),8.31-8.42(m,3H,Ar-H),7.81(d,J=6.1Hz,1H,Ar-H),7.68(m,2H,-CONH 2 ),7.30-7.40(m,2H,Ar-H),6.97(s,1H,Ar-H),4.81(t,J=8.2Hz,2H,-NHCH 2 -), 4.28 (d, J=6.1Hz, 2H, -NHCH 2 -), 2.91(t, J=8.2Hz, 2H, Ar-CH 2 -), 2.65(d, J=8.2Hz, 2H, Ar-CH 2 -), 2.03(m, 1H, -CHCO-), 1.73(d, J=8.2Hz, 4...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com