Preparation method of ethyl 3-ethoxypropionate
A technology of ethyl ethoxy propionate and ethyl acrylate, which is applied in the field of preparation of ethyl 3-ethoxy propionate, can solve the problems of easy breakage, loss of use, low conversion rate, high reaction temperature, etc., and achieve convenient The effects of continuous production, simplified separation process, and simple synthesis method
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
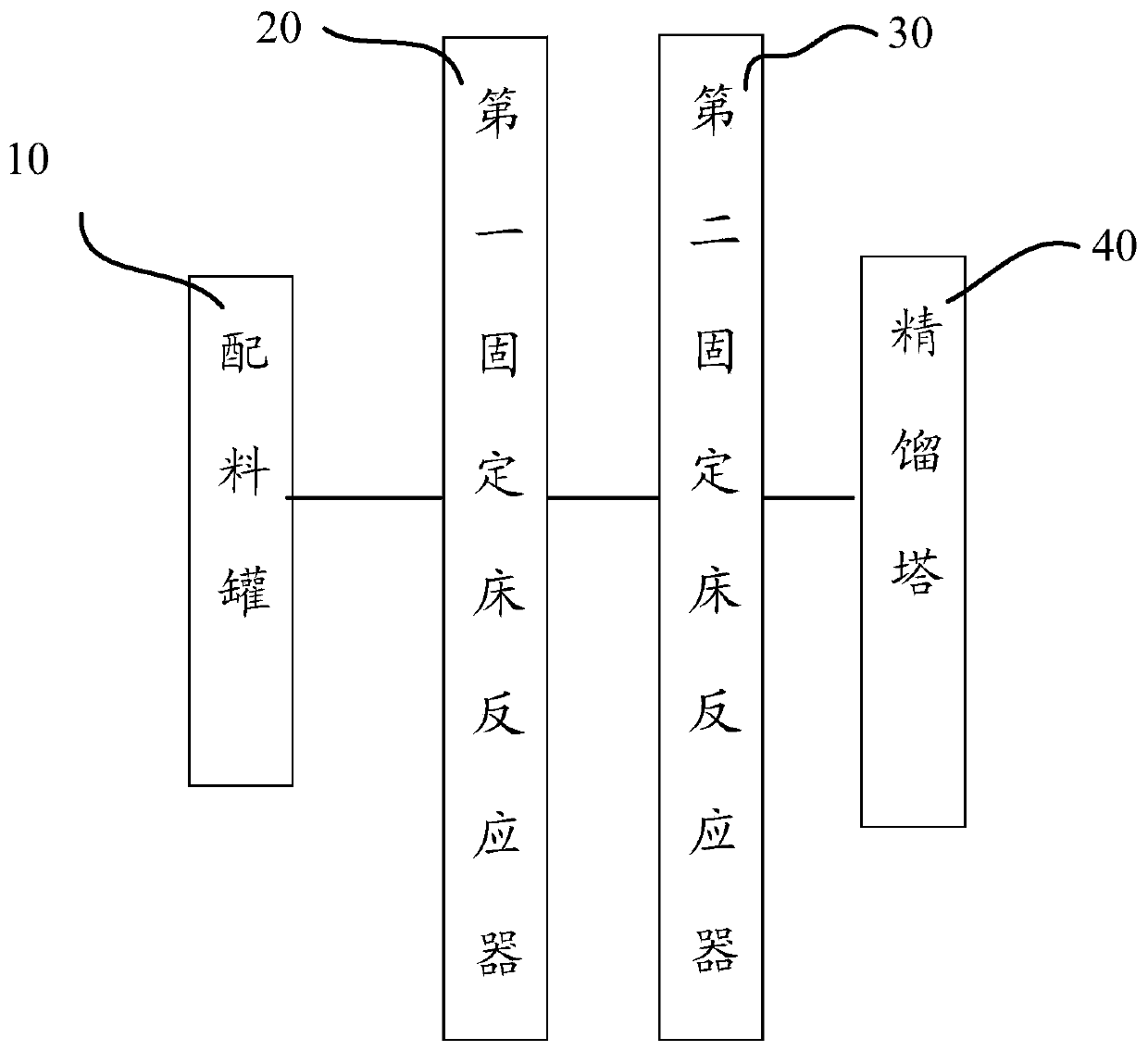
[0033] see figure 1 , the embodiment of the present invention provides a kind of preparation method of ethyl 3-ethoxypropionate, comprising the following steps:
[0034] The reaction step is to carry out addition reaction of absolute ethanol and ethyl acrylate in a molar ratio of 3-100:1 under the action of a composite catalyst, and the reaction temperature is -5 to 35°C to obtain ethyl 3-ethoxypropionate mixture of esters;
[0035] Purification step, purifying and separating the mixture containing ethyl 3-ethoxypropionate to obtain ethyl 3-ethoxypropionate with high purity;
[0036] Wherein, the acidity of the dehydrated ethanol≤10ppm; the acidity of the ethyl acrylate≤10ppm;
[0037] The dosage of the composite catalyst is 0.1% to 15% of the weight of ethyl acrylate;
[0038] The composite catalyst includes strong basic ion exchange resin.
[0039] In a specific embodiment, the composite catalyst further includes a cocatalyst and a stabilizer; wherein, the stabilizer is ...
Embodiment 1
[0064] The first fixed-bed reactor 20 is 2.5 centimeters of radius, and 25 centimeters of heights are equipped with deacidification resin; , cocatalyst and stabilizing agent), first clean the first fixed-bed reactor 20 and the second fixed-bed reactor 30 with dehydrated ethanol, to take away the points wherein, the dehydrated ethanol water content to coming out is lower than 0.5 %.
[0065] The mass ratio of dehydrated alcohol and ethyl acrylate in the batching tank 10 is 85:15; The deacidification resin consumption in the first fixed-bed reactor 20 is about 10% of ethyl acrylate, and the The dosage of composite catalyst is about 5% of that of ethyl acrylate.
[0066] The acidity of the mixed material of ethyl acrylate and dehydrated alcohol after the first fixed-bed reactor 20 is at 5-9ppm, is cooled to the temperature between 9-13 degrees; Passes into the second fixed-bed reactor by the dilution rate of 2.1 30, the reaction temperature is controlled at 26 ± 2 degrees, the ...
Embodiment 2
[0068] The difference with Example 1 is that the mass ratio of absolute ethanol and ethyl acrylate in the batching tank 10 is 85:15; the deacidification resin consumption in the first fixed bed reactor 20 is about 12% of ethyl acrylate , the composite catalyst consumption in the second fixed bed reactor 30 is about 7% of ethyl acrylate.
[0069] The acidity of ethyl acrylate and absolute ethanol mixture material after the first fixed-bed reactor 20 is 5-9ppm, is cooled to the temperature between 19-20 degrees, passes into the second fixed-bed reactor 30 by the dilution rate of 2.0 , the temperature of the second fixed-bed reactor 30 is controlled at 28 ± 2 degrees, the material coming out from the second fixed-bed reactor 30 enters the receiving tank, and the content of ethyl acrylate in the receiving tank is measured, and the material in the receiving tank is through rectification Tower 40 rectification, the EEP that obtains purity 99.8% is collected as product, calculates yi...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Bronsted acidity | aaaaa | aaaaa |
| radius | aaaaa | aaaaa |
| height | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com