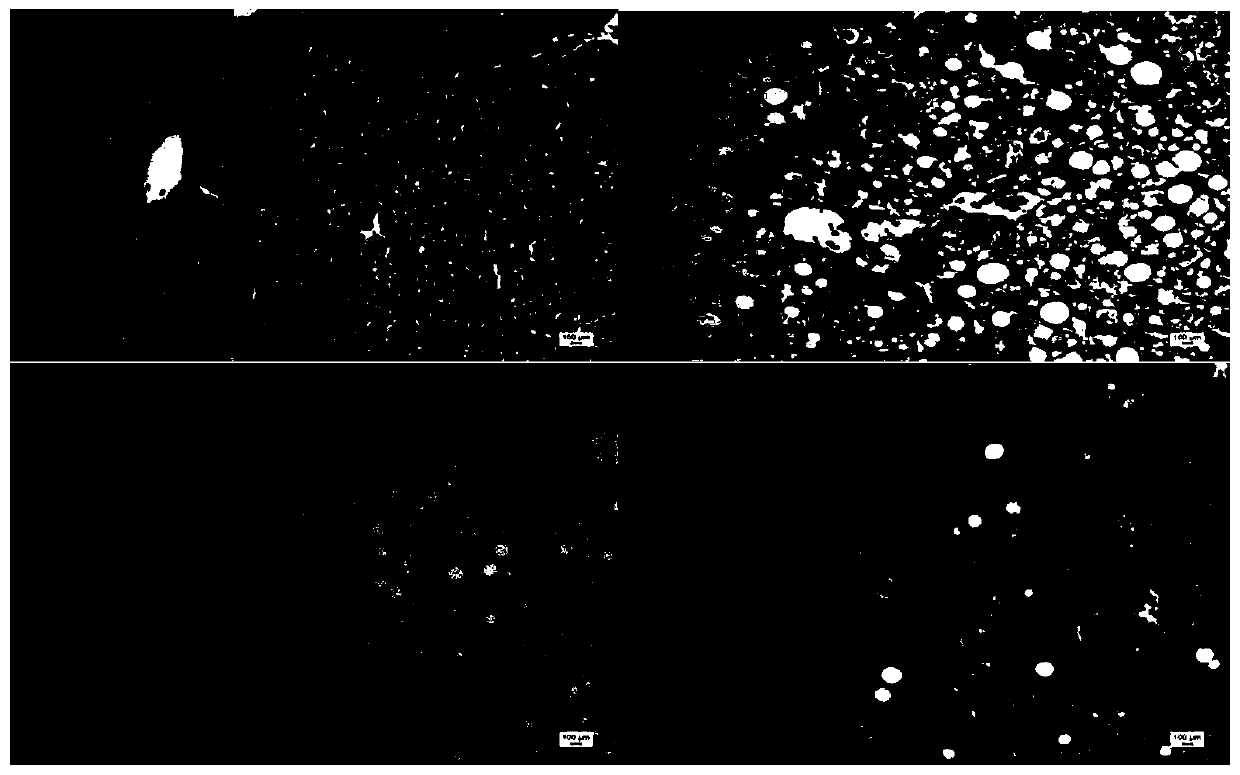
Application of Piceatannol 3'-O-glucoside in preparation of product for treating and/or preventing non-alcoholic fatty liver disease
A non-alcoholic technology of trizaperoside, which is applied in the field of application of trizaperoside in the preparation of products for the treatment and/or prevention of non-alcoholic fatty liver disease, can solve the problems of no treatment and prevention of NAFLD, and achieve a good market Prospect, improvement of hepatocyte steatosis, effect of improving cultured hepatocyte steatosis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
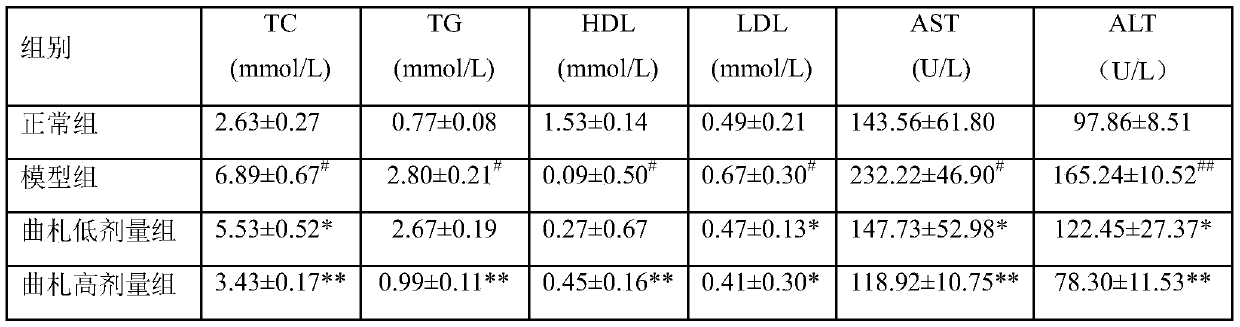
[0036] Example 1: Discussion on the protective effect of tristilbene glycosides on non-alcoholic liver cell injury
[0037] 1 Experimental materials
[0038] 1.1 Test product
[0039] Tristilbene, molecular weight 406, white crystal or crystalline powder, purity 99.6%, batch number 20120402;
[0040] 1.2 Experimental animals
[0041] Select 44 healthy male C57BL / 6 mice, weighing 19-23g, all purchased from Hunan Slack Jingda Experimental Animal Co., Ltd., experimental animal production license number: SCXK (Xiang) 2016-0002, and bred in Kunming Pharmaceutical Group Research Institute, group breeding in PVC transparent plastic boxes, each box ≤ 6, fed with the corresponding feed every day, free drinking water, and changing cages and bedding materials according to the situation. Temperature 20-25°C (daily temperature difference ≤3°C), humidity 40%-70%, lighting 12h: 12h alternating light and dark, illumination 150-300lx, noise ≤60dB, experimental animal use license: SYCK (Dian...
Embodiment 2
[0086] The tablet of this example consists of the following components: 10 g of tristilbene glycosides, 20 g of microcrystalline cellulose, 20 g of pregelatinized starch, 20 g of cross-linked polyvinylpyrrolidone, and 1 g of micropowdered silica gel.
[0087] The above-mentioned raw materials were mixed, and trizaperine tablets were prepared according to conventional methods.
Embodiment 3
[0089] The capsule of this embodiment is composed of the following components: 10 g of stilbene glycosides, 30 g of microcrystalline cellulose, 5 g of lactose, an appropriate amount of povidone K-30, and 1 g of magnesium stearate.
[0090] Mix the above-mentioned raw materials, and prepare tristilbene capsules according to conventional methods.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com