Preparation method for synthesizing pyrroloquinoline quinone by five-step method
A technology of pyrroloquinoline quinone and one-step method is applied in the field of preparation of five-step method for synthesizing pyrroloquinoline quinone, and can solve the problems of large environmental pollution, high preparation cost, difficult industrialized production and the like
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
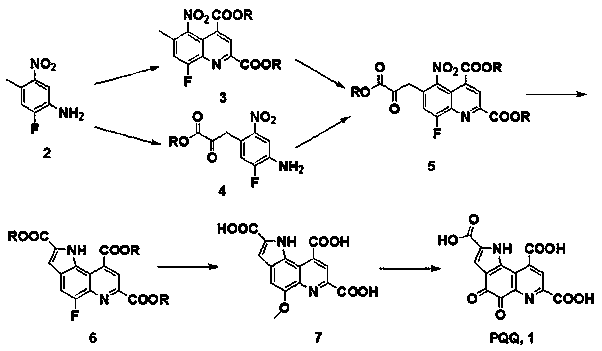
[0023] The preparation method of pyrroloquinoline quinone: 4-methyl-5-nitro-2-fluoroaniline (2) (170.14 g, 1.00 mol), 1,2-dichloroethane (1.00 L), 4-oxo Diethyl-2-enylglutarate (200.19 g, 1.00 mol) and concentrated hydrochloric acid (8 mL, 0.1 mol) were placed in a three-necked flask, and heated to reflux for 5 h. After the reaction was cooled, the liquids were separated and dried. The solvent was recovered under reduced pressure to obtain the crude product 6-methyl-5-nitro-8-fluoroquinoline-2,4-dicarboxylic acid diethyl ester (3); compound 3, diethyl oxalate (146.14 g, 1.00 mol), sodium carbonate (212.00 g, 2.00 mol) and tetrahydrofuran (1.00 L) were placed in a three-necked flask, heated to reflux for 5 hours, after the reaction was cooled, filtered, and the solvent was recovered under reduced pressure to obtain the crude product 6-(2-ethoxy Carbonyl-2-oxoethyl)-5-nitro-8-fluoroquinoline-2,4-dicarboxylate (5); compound 5, 5% palladium on carbon (2 g) and methanol (1.00 L) w...
Embodiment 2
[0025] The preparation method of pyrroloquinoline quinone: 4-methyl-5-nitro-2-fluoroaniline (2) (170.14 g, 1.00 mol), dichloromethane (1.00 L), 4-oxo-2-ene Dimethyl glutarate (172.14 g, 1.00 mol) and 5 M dilute sulfuric acid (20 mL, 0.1 mol) were placed in a three-neck flask, heated to reflux for 5 h, after the reaction was cooled, separated, dried, and recovered under reduced pressure solvent to obtain crude 6-methyl-5-nitro-8-fluoroquinoline-2,4-dicarboxylic acid dimethyl ester (3); compound 3, dimethyl oxalate (118.09 g, 1.00 mol), Potassium carbonate (276.00 g, 2.00 mol) and 2-methyltetrahydrofuran (1.00 L) were placed in a three-necked flask, heated to reflux for 5 h, after the reaction was cooled, filtered, and the solvent was recovered under reduced pressure to obtain the crude product 6-(2-methanol Oxycarbonyl-2-oxoethyl)-5-nitro-8-fluoroquinoline-2,4-dicarboxylic acid dimethyl ester (5); compound 5, Raney nickel (5 g) and methanol (1.00 L) was placed in a high-pressu...
Embodiment 3
[0027]The preparation method of pyrroloquinoline quinone: 4-methyl-5-nitro-2-fluoroaniline (2) (170.14 g, 1.00 mol), benzene (1.00 L), 4-oxo-2-pentadiene Acid dibenzyl ester (324.33 g, 1.00 mol) and methanesulfonic acid (9.61 g, 0.1 mol) were placed in a three-necked flask, heated to reflux for 5 h, after the reaction was cooled, washed with water, separated, and dried to obtain 6-methyl Dibenzyl-5-nitro-8-fluoroquinoline-2,4-dicarboxylate (3) in benzene; compound 3 in benzene, dibenzyl oxalate (270.28 g, 1.00 mol) and Sodium hydroxide (40.00 g, 1.00 mol) was placed in a three-necked flask, and heated to reflux for 5 h. After the reaction was cooled, the solvent was recovered under reduced pressure to obtain the crude product 6-(2-benzyloxycarbonyl-2-oxoethyl) -Dibenzyl 5-nitro-8-fluoroquinoline-2,4-dicarboxylate (5); put compound 5, 20% platinum on carbon (5 g) and ethanol (1.00 L) in an autoclave , filled with hydrogen, kept the pressure of 5 atmospheres, heated to 50 ℃, re...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com