Preparation method of ultraviolet light absorber 2-phenylbenzimidazole
A technology of phenylbenzimidazole and absorbent, which is applied in the field of preparation of ultraviolet absorbent 2-phenylbenzimidazole, can solve the problems of polluted environment, low product yield, large amount of waste acid, etc., and achieves simplified preparation process, reduce the temperature of sulfonation reaction, and ensure the effect of high purity
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
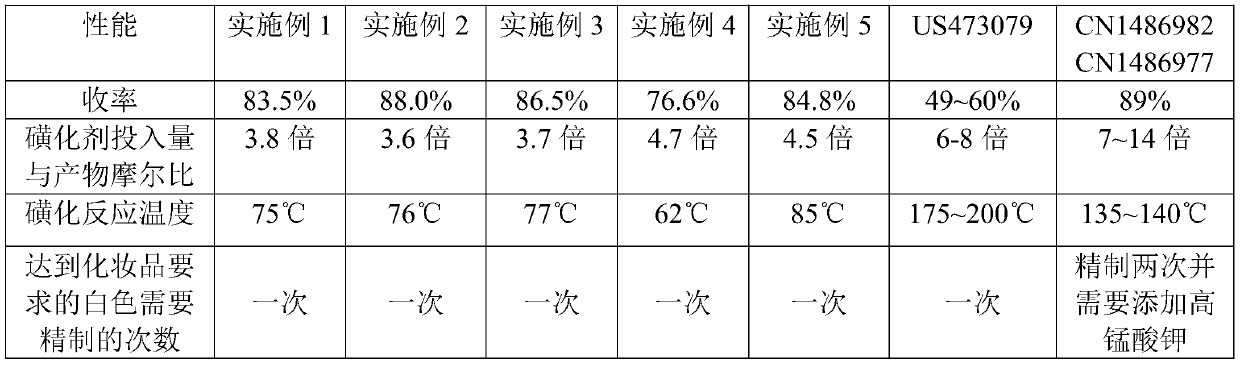
Embodiment 1
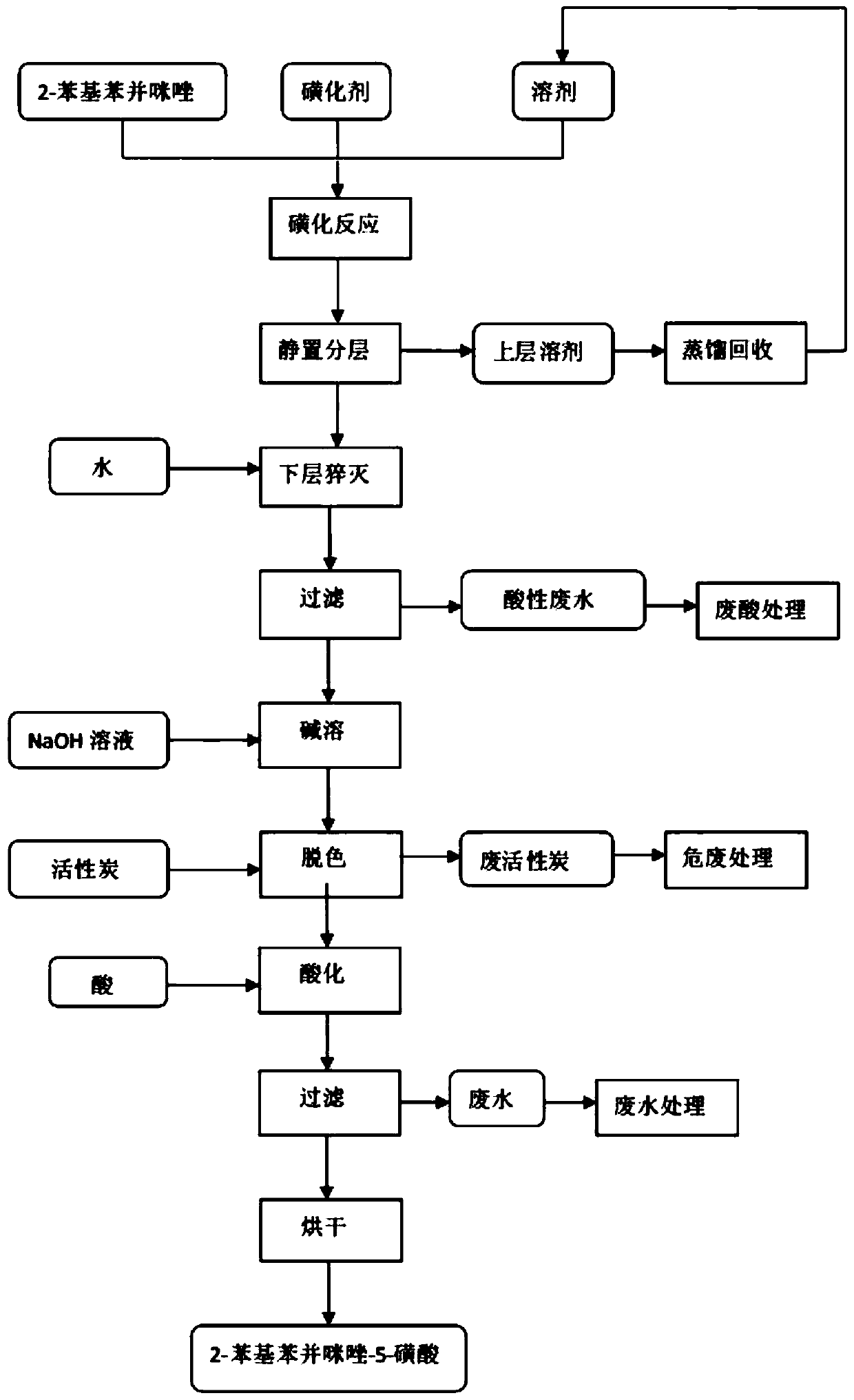
[0028] Such as figure 1 Shown, a kind of preparation method of ultraviolet absorber 2-phenylbenzimidazoles specifically comprises the following steps: 157g (1.35mol) chlorosulfonic acid is slowly dripped in the sulfuric acid of 180g98% in stirring, mix uniformly and set aside. Put 194g (1.0mol) of 2-phenylbenzimidazole and 1000g of dichloroethane into a 2L reaction flask, stir and raise the temperature to 75°C, drop the above-mentioned mixed acid into the reaction flask for reaction, and the dropping time is 3 hours. After the dropwise addition, continue the insulation reaction for 3 hours, and absorb the hydrogen chloride gas produced in the reaction process with lye.
[0029] After the reaction, stop stirring and let it stand for stratification. The light yellow solution in the upper layer is dichloroethane, which can be directly used in the next batch of reactions without distillation to make up the lost amount. The dark liquid in the lower layer was dropped into 2000L of ...
Embodiment 2
[0032] Slowly drop 140g (1.2mol) of chlorosulfonic acid into 200g of 100% sulfuric acid under stirring, mix well and set aside. Put 194g (1.0mol) of 2-phenylbenzimidazole and 1000g of dichloroethane into a 2L reaction flask, stir and raise the temperature to 76°C, drop the above-mentioned mixed acid into the reaction flask for reaction, and the dropping time is 3 hours. After the dropwise addition, continue the insulation reaction for 3 hours, and absorb the hydrogen chloride gas produced in the reaction process with lye.
[0033] After the reaction, stop stirring and let it stand for stratification. The light yellow solution in the upper layer is dichloroethane, which can be directly used in the next batch of reactions without distillation to make up the lost amount. The dark liquid in the lower layer was dropped into 2000L of vigorously stirred water, and the hydrolysis temperature was controlled at 10°C. After separation by filtration, the obtained white solid is the crude...
Embodiment 3
[0036] Put 194g (1.0mol) of 2-phenylbenzimidazole, 900g of dichloroethane recovered by layering in the previous batch, 100g of fresh dichloroethane, and 180g of 98% sulfuric acid into a 2L reaction flask, and stir to raise the temperature to 77°C. Take by weighing 163g (1.4mol) chlorosulfonic acid, drop in the reaction flask and react, the dropwise addition time is 3 hours, continue insulation reaction for 3 hours after dropwise addition, absorb the hydrogen chloride gas that reaction process produces with lye.
[0037] After the reaction, stop stirring and let it stand for stratification. The light yellow solution in the upper layer is dichloroethane, which can be directly used in the next batch of reactions without distillation to make up the lost amount. The dark liquid in the lower layer was dropped into 2000L of vigorously stirred water, and the hydrolysis temperature was controlled at 20°C. After separation by filtration, the obtained white solid is the crude product of ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com