Bivalent inactivated vaccine for pigs, preparation method and application thereof
A dual inactivated vaccine and inactivation technology, applied in botany equipment and methods, biochemical equipment and methods, applications, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
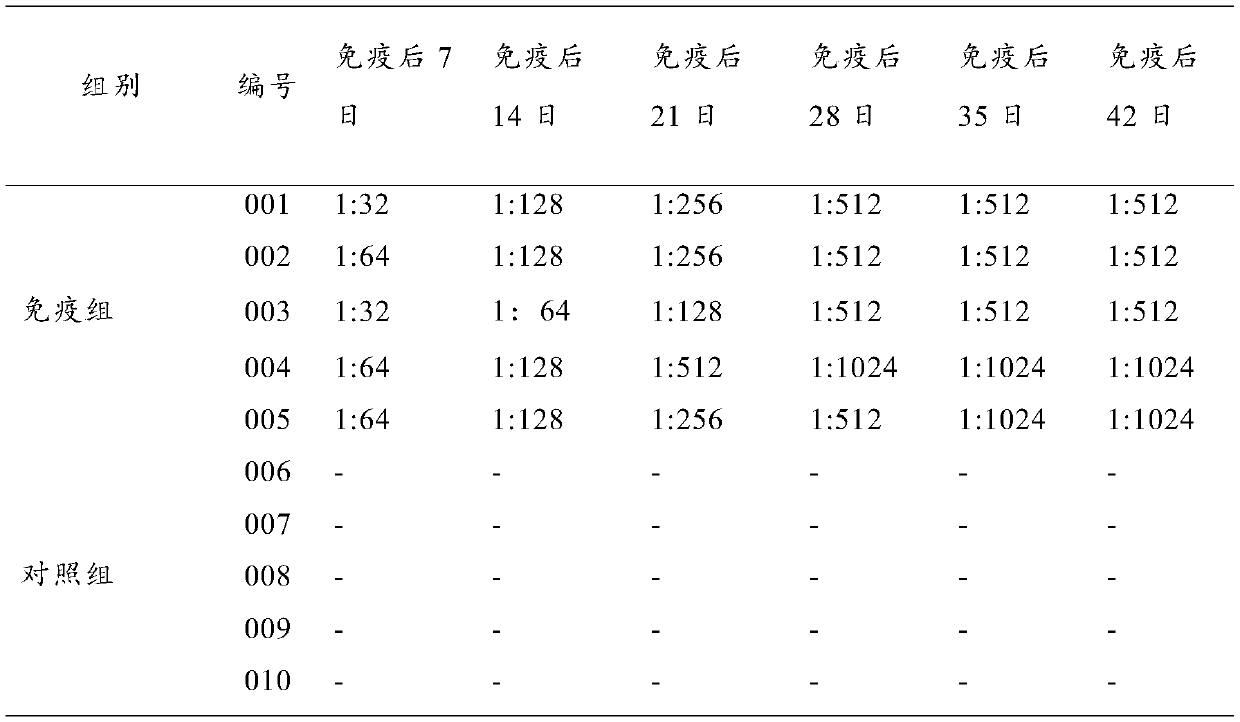
[0022] Embodiment 1 pig uses the preparation of double inactivated vaccine
[0023] 1 poison seed
[0024] Porcine Seneca virus, its microbial preservation number is: CGMCC No.18851; classification name: Seneca virus; preservation unit: General Microbiology Center of China Committee for the Collection of Microorganisms; preservation time is October 29, 2019; preservation Address: No. 3, Yard No. 1, Beichen West Road, Chaoyang District, Beijing.
[0025] 2 test method
[0026] 2.1 Preparation of porcine foot-and-mouth disease baculovirus
[0027] 2.1.1 Design and synthesis of FMDV-vp1 gene
[0028] The O-type FMDV VP1 gene (SEQ ID NO.1) was codon-optimized to obtain the optimized sequence (SEQ ID NO.2), and EcoRI and Hind III restriction sites were added to both ends of the optimized sequence and cloned into the pFastBacⅠ vector , forming heavy pFB-FMDV-vp1.
[0029] 2.1.2 Construction and preparation of recombinant shuttle bacmid rBacmid-FMDV-vp1
[0030] 2.1.2.1 Constru...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com