Application of cobratide preparation in preparation of medicine for treating postherpetic neuralgia
A technology for postherpetic neuralgia and herpes zoster, which can be used in drug combinations, nervous system diseases, antiviral agents, etc., and can solve problems that have not been reported
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
experiment example 1
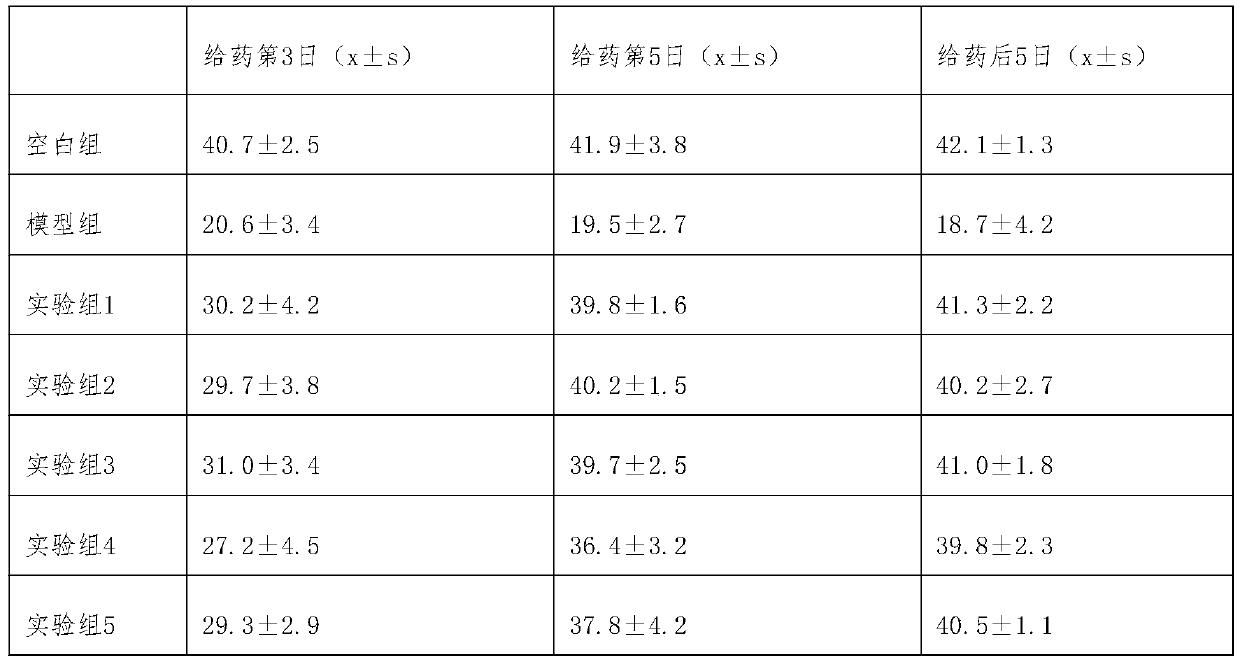
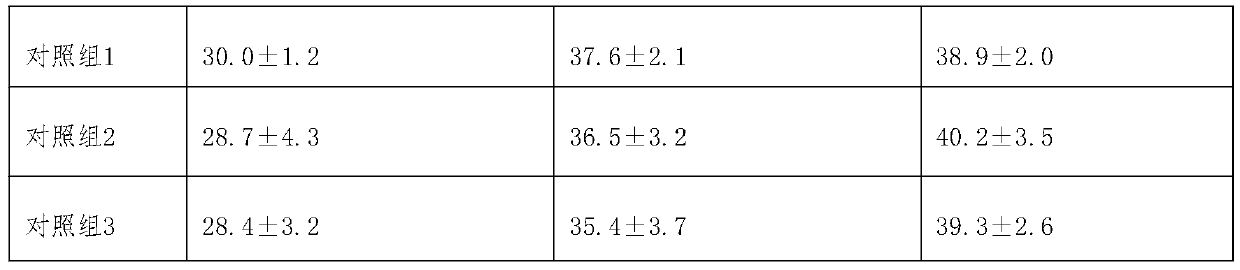
[0017] Experimental Example 1 Experimental Study on Pharmacodynamic Evaluation and Mechanism Analysis of Cobotide Preparations in Treating Postherpetic Neuralgia
[0018] 1. Test material
[0019] Group 1: Cobotide injection on the market, provided by Yunnan Nanzhao Pharmaceutical Co., Ltd., specification: 2ml: 70μg; usage and dosage: intramuscular injection, 4ml each time, twice a day, and the injection time should be more than 6 hours .
[0020] Group 2: Cobotide oral tablets, made according to the existing disclosed technical scheme, specifications 280μg / 0.25g tablet; usage and dosage: 1 tablet each time, once a day.
[0021] Group 3: Cobotide enteric-coated tablets, made according to the existing disclosed technical scheme, specifications: 280 μg / 0.25g / vegetarian tablet, usage and dosage: 1 tablet per day, once a day.
[0022] Group 4: Cobotide rectal administration suppository, made according to the existing disclosed technical scheme, specification: 140 μg / 2g supposito...
experiment example 2
[0040] Experimental Example 2 Animal Toxicity Test
[0041] 80 SPF grade SD rats, male and female half and half, body weight 180-220g; divided into 8 groups, each group of 5 male and female; according to the administration method of experimental group 1-5 and matched group 1-3 in experimental example 1 medicine. Continuously administered for 30 days, during the period of administration and 5 days after drug withdrawal, the growth status and activity diet of the mice were observed, and the hematology, blood biochemistry, organ tissue structure and urine routine were identified. The results showed that all SD rats survived healthy without any side effects; the anatomical observations of blood, liver function, and the state of various organs and tissues showed no difference compared with normal SD rats. Experiments have proved that the cobotide preparation provided by the present invention is safe and reliable for administration.
[0042] 3. Conclusion
[0043] Postherpetic ne...
PUM
| Property | Measurement | Unit |
|---|---|---|
| molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com