Strain for preventing and treating metabolic diseases and application for strain
A technology for metabolic diseases and bacterial strains, which is applied in the field of microorganisms in the field of biotechnology, can solve the problems of Akkermansia strains' fermentation performance, storage stability, acid resistance and bile salt resistance, and the effect of treating metabolic diseases is not particularly ideal, to achieve Effects of lowering blood sugar, reducing visceral fat content, and delaying weight gain
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0029] Embodiment 1: the screening of bacteria
[0030] 1. Medium
[0031] Separation medium: 0.4g KH 2 PO 4 ; 0.53g Na 2 HPO 4 ; 0.3 g NH 4 Cl; 0.3g NaCl; 0.1gMgCl 2 .6H 2 O; 0.11 g CaCl 2 ; 0.5mg resazurin; 4g NaHCO 3 ; 0.25g Na 2 S.7–9H 2 O; 0.25% mucin.
[0032] Fermentation Medium 1: Brain Heart Infusion Broth (Brain Heart Infusion Powder, BHI)
[0033] Fermentation medium 2: 5g tryptone, 5g soytone, 5g beef powder, 5g yeast powder, 4g glucose, 3gNaCl, 1.5g MgSO 4 , Na 2HPO 4 , KH 2 PO 4 , water 1L, pH=7.2.
[0034] Separation and fermentation medium were autoclaved at 121°C for 15 minutes for later use.
[0035] 2. Isolation and identification of strains
[0036] ①The prepared mucin separation medium is placed in a serum bottle (filling volume 10 / 30mL)
[0037] ② Take 0.5g of feces from healthy adults and place them in sterile PBS phosphate buffered saline solution to disperse evenly. Dilute the feces in a 10-fold gradient in PBS solution in turn, and...
Embodiment 2
[0042] Embodiment 2: Akkermansia fermentation
[0043] ① Akkermansia was inoculated on brain heart powder agar with a three-section line, and cultured anaerobically at 37°C for 48 hours.
[0044] ②Pick a single colony of Akkermansia from the agar plate and inoculate it into 15mL brain heart extract powder liquid medium, and culture it anaerobically at 37°C for 24h.
[0045] ③Transfer 10mL of fermentation broth from a 15mL anaerobic tube to 200mL of brain heart extract powder liquid medium, and culture at 37°C for 24h in anaerobic conditions.
[0046] ④ 200mL of Akkermansia fermented liquid was inoculated in the brain heart extract powder liquid medium, the volume of the 5L fermentation tank was 4L, and the anaerobic gas was nitrogen and carbon dioxide mixed binary gas (N 2 :CO 2 =9:1), the stirring speed was 100r / min, the temperature was 37°C, and the anaerobic culture was carried out for 24h.
Embodiment 3
[0047] Embodiment 3: Akkermansia fermentation
[0048] ① A single colony of W03 and standard strain ATCC BAA835 (purchased from the American Type Culture Collection) was inoculated in 15 mL of fermentation medium 2, and cultured anaerobically at 37°C for 24 hours.
[0049] ②Transfer 10mL of fermentation broth from a 15mL anaerobic tube to 200mL of fermentation medium 2, and incubate at 37°C for 24 hours anaerobically.
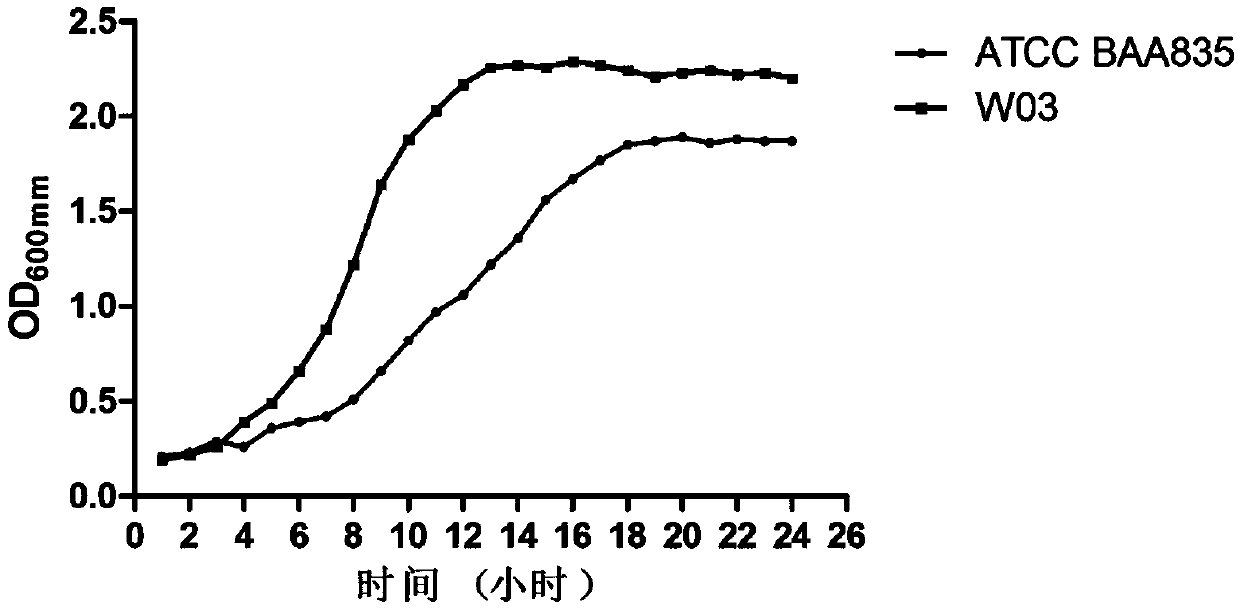
[0050] ③ 200mL of Akkermansia fermentation broth was inoculated in fermentation medium 2, the volume of the 5L fermentation tank was 4L, and the anaerobic gas was nitrogen and carbon dioxide mixed binary gas (N 2 :CO 2 =9:1), the stirring speed is 100r / min, the temperature is 37°C, the process control pH is 6.5, and the anaerobic culture is carried out until the OD does not change.
[0051] Result: if figure 1 As shown, the W03 bacteria fermented for 13 hours with a maximum OD of 2.26 (2.13×10 9 CFU / mL) reached the stable stage, and the maximum OD of ATCC ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com