Preparation for inhibiting adverse reactions of opioid analgesic drugs and application of preparation
A technology of adverse reactions and opioids, applied in the field of medicine, can solve the problems of patient dependence, large dose, poor effect, etc., and achieve the effect of reducing treatment costs and opioid dependence
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0041] Example 1: Simultaneous administration of 17-AAG with morphine, experiment and results of acute administration
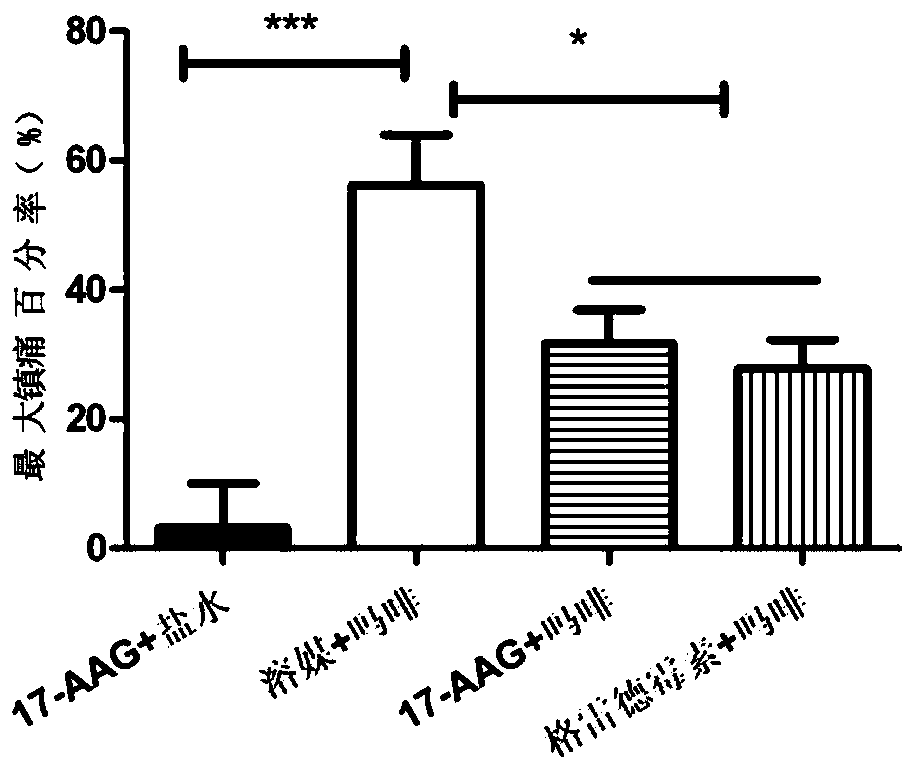
[0042] A total of 40 C57 / B6 mice were randomly divided into 4 groups. The basic pain range of the animals was measured on a hot plate (55°C) before administration, and then 5 microliters of 17-AAG (10nmol) and solvent (solvent) were administered intracerebroventricularly. ), 17-AAG (10nmol) and geldanamycin (10nmol), 15 minutes later, subcutaneous administration of normal saline (10ml / kg), morphine (Mor, 10mg / kg), morphine (Mor, 10mg / kg ), morphine (Mor, 10mg / kg), and the pain zone of the animals was measured on a hot plate 30 minutes after administration. Maximum analgesic percentage (%MPE)=(pain domain after administration-pain domain before administration) / (60-pain domain before administration)×100%. From Table 1 and figure 1 It can be seen that 17-AAG has no analgesic effect on mice, and morphine has obvious analgesic effect on mice. 17-AAG (10nmol) and...
Embodiment 2
[0047] Example 2 Simultaneous administration of 17-AAG with morphine, experiment and results of chronic administration
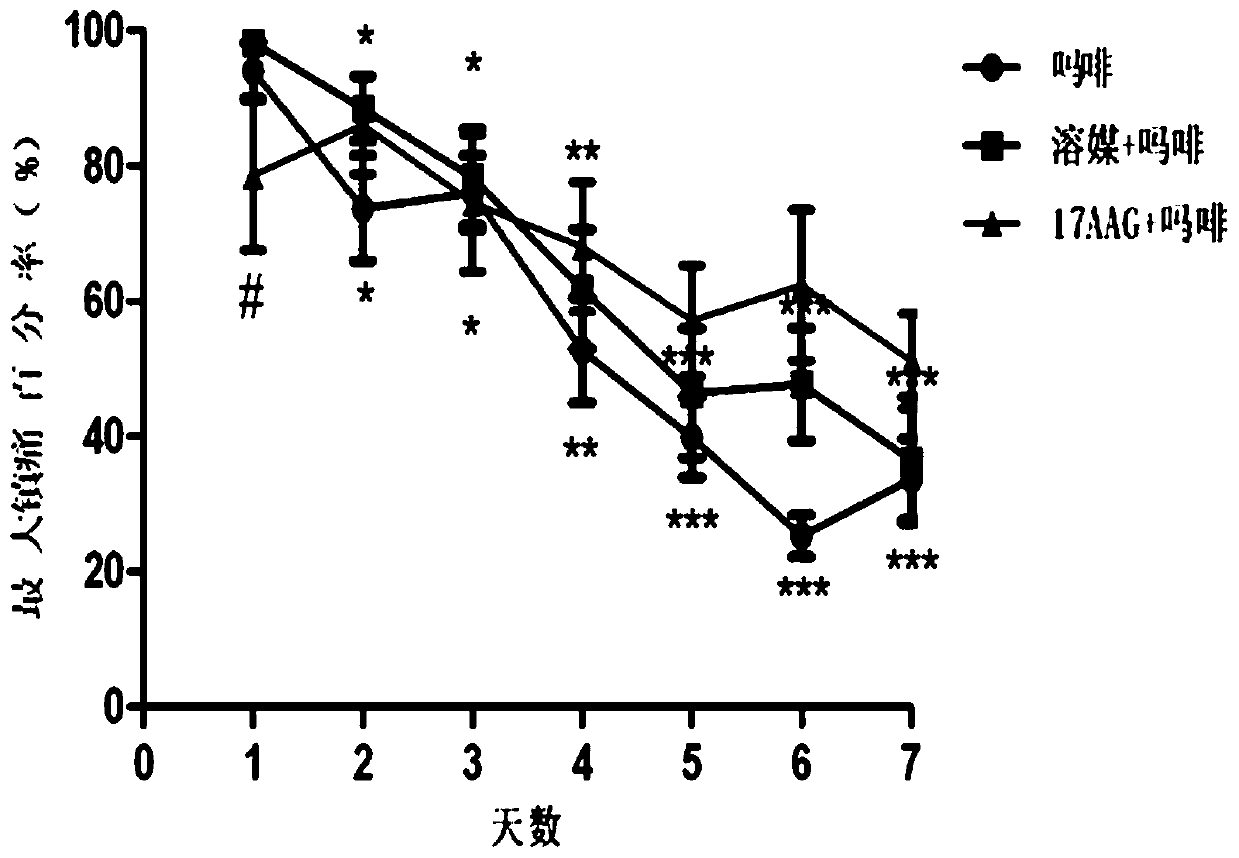
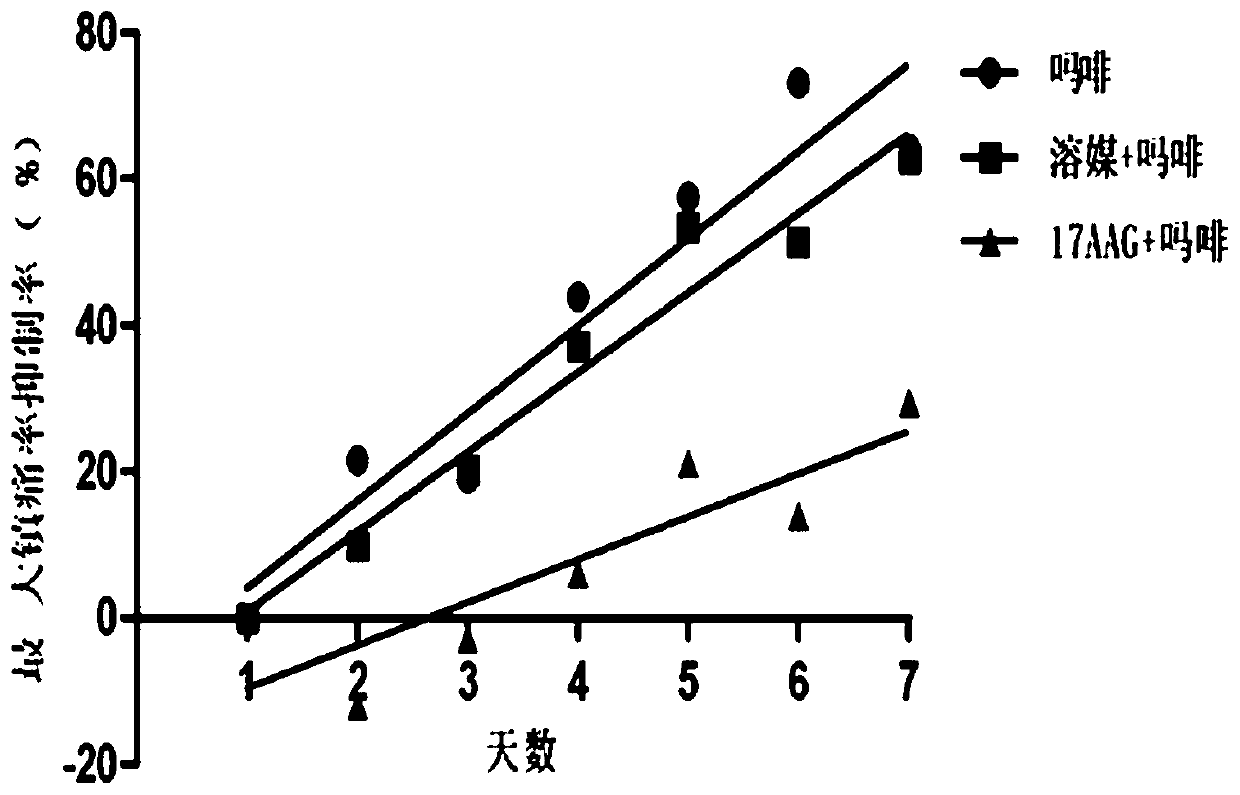
[0048] A total of 30 C57 / B6 mice were raised in the experimental environment for 2-3 days, randomly divided into 3 groups, administered twice a day, starting at 8:00 in the morning, and the animals were measured on a hot plate (55°C) before administration. For the basic pain zone, 5 microliters of solvent and 17-AAG (10nmol) were administered into the ventricle, and morphine (Mor, 100mg / kg) was administered subcutaneously 15 minutes later, and the pain zone was measured after 30 minutes of morphine administration. At 6:00 p.m., the animals in the three groups were only given morphine (100 mg / kg), continuously administered and measured for 7 days, and naloxone (10 mg / kg) was injected intraperitoneally 2 hours after the last administration, and immediately put Enter the withdrawal observation box, observe the withdrawal signs (withdrawl signs) such as jumping,...
Embodiment 3
[0056] Example 3 17-AAG co-administered with morphine, experiment and results of influence on mental dependence
[0057] The experimental timing design of chronic administration of 17-AAG on morphine conditioned place preference see Figure 5 A total of 50 C57 / B6 mice were used, and the animals were raised in the experimental environment for 2-3 days. The pre-adaptation period (d1-d3): 3 days, once a day, 15 minutes each time, and two times within 15 minutes after recording. Side residence time, the average value of d2 and d3 was used as the pre-test result, and unqualified animals were screened out.
[0058] Criteria for animal exclusion: the difference between the residence time of all animals in the two sides of the black box and the white box is more than 100s. It is considered that there is a natural bias, and the individuals with similar residence times in the two sides of the box are eliminated; The difference between the residence time in the box does not exceed 100s,...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com