Preparation and detection method of mercury ion electrochemical biosensor
A biosensor and detection method technology, applied in the field of bioelectrochemistry, can solve the problems of high detection cost, time-consuming operation, complicated instruments, etc., and achieve the effects of good selectivity, convenient portability and simple operation.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
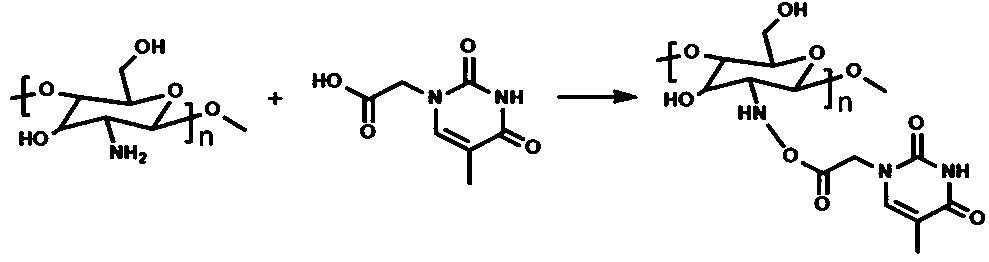
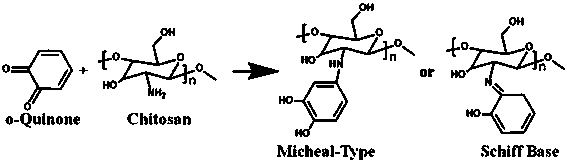
[0047] Embodiment 1: Step 1. Weigh 0.1g chitosan and stir it and dissolve it in 10ml of dilute hydrochloric acid aqueous solution; configure concentrated NaOH aqueous solution, slowly add it to the chitosan aqueous solution, white solids are precipitated, and the precipitated The white solid was centrifuged and washed several times with absolute ethanol and stored in absolute ethanol; respectively weighed 0.55g Tacetic acid, 0.57g EDC•HCl and 0.345g NHS, added 20ml of absolute ethanol to it, and stirred in an ice bath And react for 1h; add the absolute ethanol solution of chitosan to the activated T-acetic acid solution, first stir in ice bath for 30min, then react at room temperature for 24h; centrifuge and wash with absolute ethanol several times, and dry Obtain CsT compound, the reaction structural formula of chitosan and thymine is as figure 1 shown.
[0048] Step 2: Put the gold electrode on the polishing cloth of 1.0, 0.3, 0.05μm respectively with 1.0, 0.3, 0.05μm Al 2...
experiment example (1)
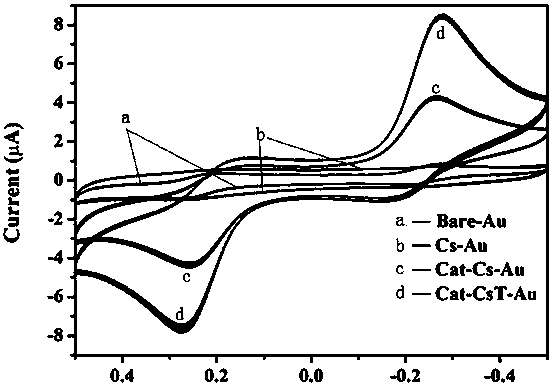
[0080] Experimental example (1): In the presence of 50 μmol / L Fc and Ru 3+ In the PBS (0.1M, pH=7.2) solution, the redox currents of bare Au, Cs-Au, Cat-Cs-Au and Cat-CsT-Au modified electrodes were measured by CVs method, and the results were as follows image 3 Shown (A: T-acetic acid concentration gradient UV-visible absorption spectrum; B: T-acetic acid standard curve and linear regression equation). After modifying Cs, since Cs is non-conductive and is an insulating film, it is observed from the figure that there is almost no peak on the Cs-Au modified electrode, indicating that Cs is modified to the surface of the Au electrode. Subsequently, Cat was modified on the surface of the Cs-Au electrode. Due to the electrochemical activity of Cat, Cat-CsT has redox activity, which can interact with Fc-Ru 3+ Electron transfer occurs between, and the figure shows a strong Fc oxidation peak and Ru 3+ Reduction peak, amplified Fc oxidation peak current and Ru 3+ The peak current ...
experiment example (2)
[0081] Experimental example (2): Weigh 1 mg of T-acetic acid and dissolve it in 5 ml of deionized water to prepare an aqueous solution with a concentration of 0.2 mg / ml, and then dilute the T-acetic acid aqueous solution by 1000 times, 800 times, 600 times, 400 times, 200 times, 100 times, 80 times, 60 times, 40 times, 20 times, the concentration is 0.0002g / L, 0.00025g / L, 0.00033g / L, 0.0005g / L, 0.001g / L, 0.002g / L, 0.0025g / L, 0.0033g / L, 0.005g / L, 0.01g / L, and measure its absorbance of ultraviolet and visible light, such as Pic 4-1 , along the direction of the arrow, the concentration of T-COOH is successively 54 μM, 27 μM, 18 μM, 14 μM, 11 μM, 5.4 μM, 2.7 μM, 1.8 μM, 1.4 μM, 1.1 μM; draw a standard curve, Pic 4-1 , and calculate the linear regression equation: y=0.04797x+4.93397, the correlation coefficient is 0.998. Pic 4-1 , the thymine grafting rate of 75%CsT is 30.9%, Figure 4-2 , 95% CsT thymine grafting rate was 47.6%.
[0082] Then respectively measure the UV-vis...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
| degree of deacetylation | aaaaa | aaaaa |
| degree of grafting | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com