Preparation method of deuterated chemical
A chemical, deuterated technology, applied in electrolytic components, electrolytic organic production, electrolytic process, etc., can solve the problem of low selectivity, achieve mild reaction conditions, controllable number, and reduce production costs
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
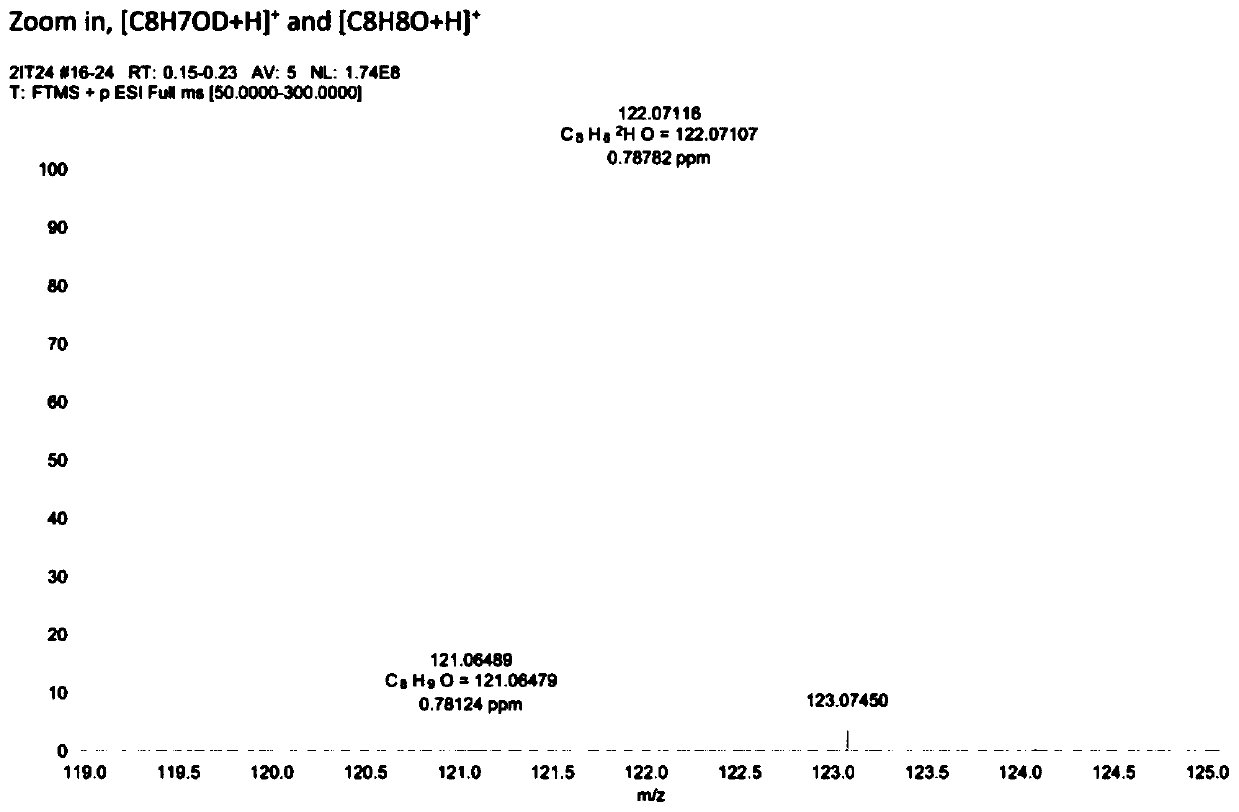
[0052] Example 1: Using deuterium water as a deuterium source, the electrocatalytic debromination and deuterium addition of 2-bromoacetophenone.
[0053]
[0054] Weigh 0.3mmol of 2-bromoacetophenone, 6mg of palladium acetylacetonate catalyst, 7.5mL of deuterium water, and 7.5mL of acetonitrile, respectively, and add them into a 30mL reaction bottle, replace the reaction system with argon protection state, and then place the graphite electrode in In the reaction bottle, set the voltage to 3.0V, turn off the power after 4 hours of reaction, and dilute the reaction mixture with 15mL CH 2 Cl 2 Extraction, the extract was dried with anhydrous sodium sulfate, the solvent was removed in vacuum, and the target product was separated by column chromatography. The high-resolution mass spectrum of the obtained product is shown in figure 1 , the resulting product was tested by GC-MS, HRMS, 1 HNMR, C-NMR, FT-IR and other tests confirmed that the yield of the reaction was 89%, and the...
Embodiment 2
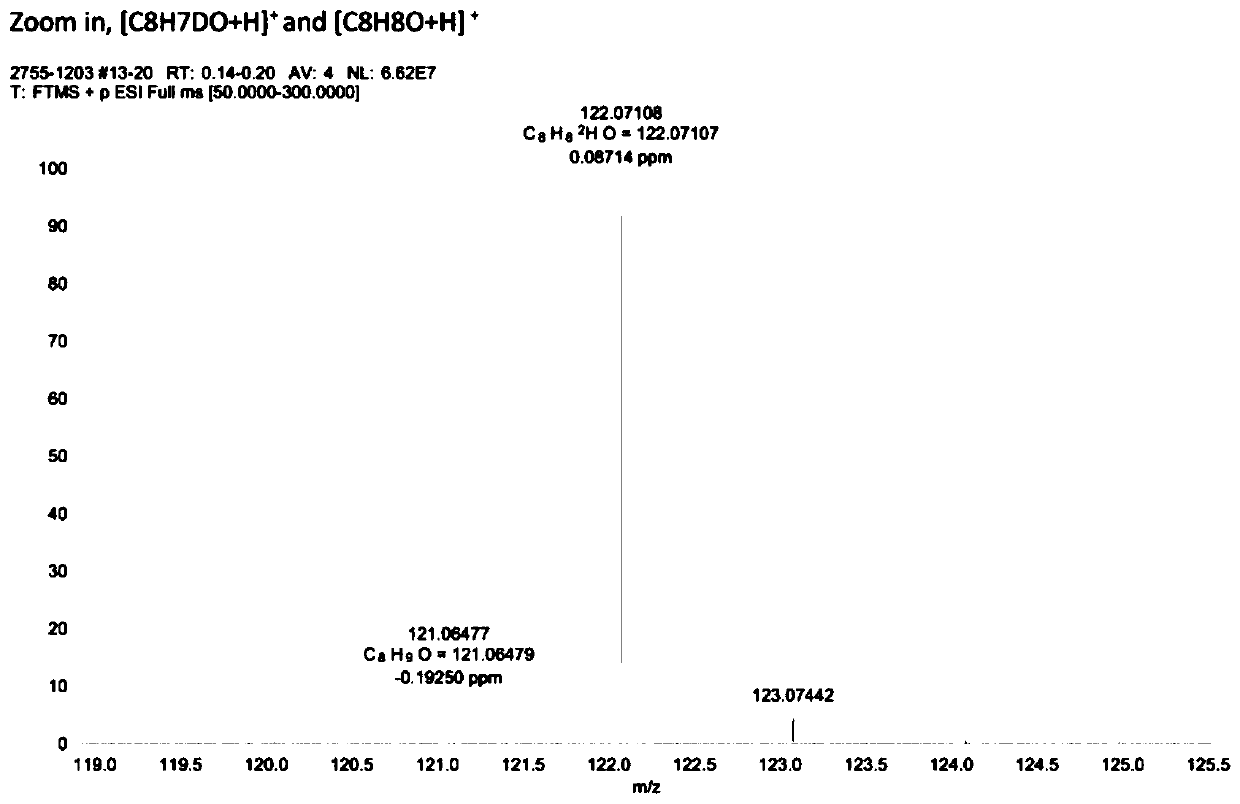
[0055] Example 2: Using deuterium water as a deuterium source, the electrocatalytic debromination and deuterium addition reaction of 3-bromoacetophenone.
[0056]
[0057] Weigh 0.3mmol of 3-bromoacetophenone, 6mg of palladium acetylacetonate catalyst, 7.5mL of deuterium water, and 7.5mL of acetonitrile respectively, and add them into a 30mL reaction bottle, replace the reaction system with argon protection state, and then place the graphite electrode in In the reaction bottle, set the voltage to 3.0V, turn off the power after 4 hours of reaction, and dilute the reaction mixture with 15mL CH 2 Cl 2 Extraction, the extract was dried with anhydrous sodium sulfate, the solvent was removed in vacuum, and the target product was separated by column chromatography. The high-resolution mass spectrum of the obtained product is shown in figure 2 , the resulting product was tested by GC-MS, HRMS, 1 Tests such as HNMR, C-NMR, and FT-IR confirmed that the yield of the reaction was 8...
Embodiment 3
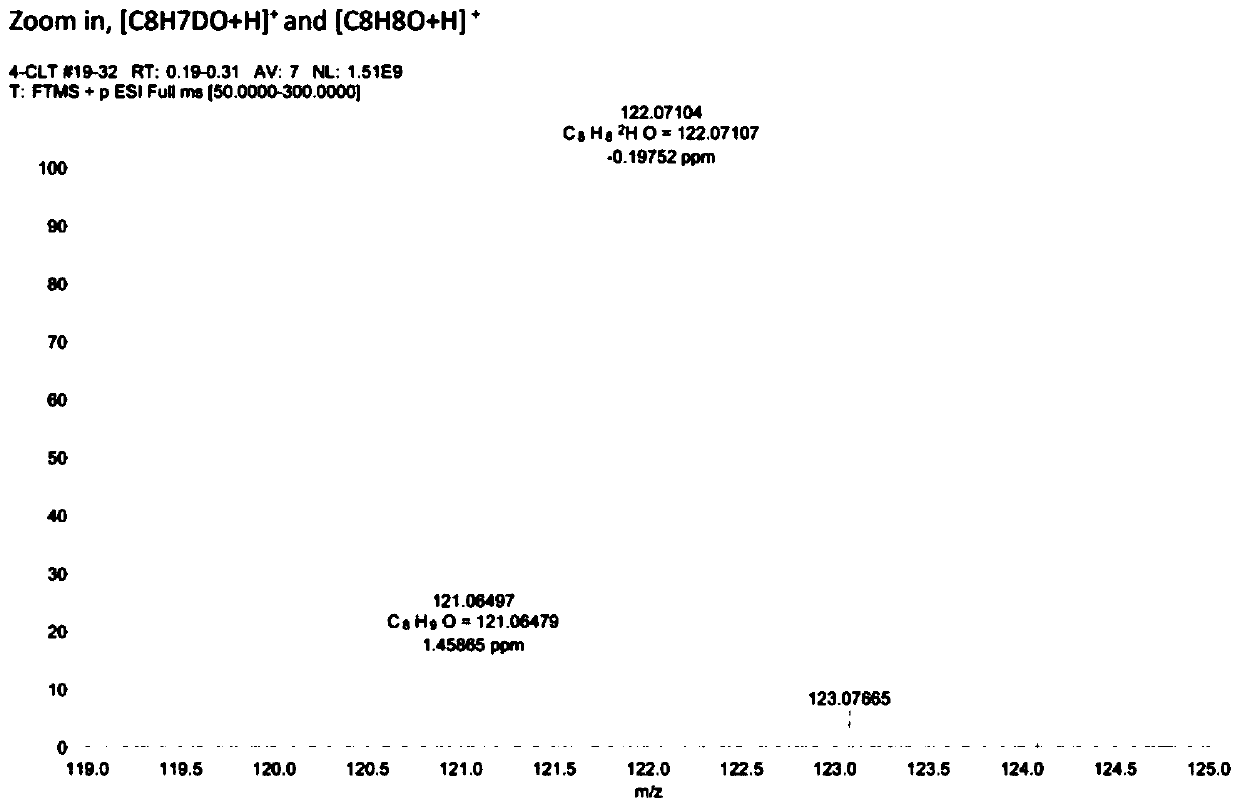
[0058] Example 3: Using deuterium water as a deuterium source, the electrocatalytic debromination and deuterium addition of 4-bromoacetophenone.
[0059]
[0060] Weigh 0.3mmol of 4-bromoacetophenone, 6mg of palladium acetylacetonate catalyst, 7.5mL of deuterium water, and 7.5mL of acetonitrile respectively, and add them into a 30mL reaction bottle, replace the reaction system with argon protection state, and then place the graphite electrode in In the reaction bottle, set the voltage to 2.6V, turn off the power after 4 hours of reaction, and dilute the reaction mixture with 15mL CH 2 Cl 2 Extraction, the extract was dried with anhydrous sodium sulfate, the solvent was removed in vacuum, and the target product was separated by column chromatography. The high-resolution mass spectrum of the obtained product is shown in image 3 , the resulting product was tested by GC-MS, HRMS,1 Tests such as HNMR, C-NMR, and FT-IR confirmed that the yield of the reaction was 88%, and the ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com