Application of Liuling Jiedu Pill in preparing drugs for preventing and treating cold diseases
A technology of detoxification pills and medicines, which is applied in the direction of drug combinations, respiratory diseases, and medical raw materials derived from molluscs. It can solve problems such as dry mouth, nausea, and lethargy, and achieve the effect of expanding clinical indications
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0025] Example 1 Determination of the median lethal titer (TCID 50 ) of influenza virus.
[0026] 1. Method.
[0027] Divide dog kidney cells (MDCK) into 3×10 4 cells / well, inoculated into 96-well plates, placed at 37°C, 5% CO 2 Cultivate until the monolayer grows. Add 10-fold diluted influenza virus solution, 100 μL / well, and incubate at 37°C for 2 hours; discard the supernatant, add fresh culture medium, 100 μL / well, observe the results after 2 days, and record the CPE.
[0028] Reed-Muench was used to calculate the half lethal titer (TCID 50) of the virus [refer to Reed LJ, MuenchH. Asimple method of estimating fifty percent endpoints. Am J Hyg, 1938, 27(3): 493-497].
[0029] 2. Results.
[0030] The median lethal titer of the amplified PR8 subtype influenza virus was detected to be 10 by Reed-Muench methodology 7.75 TCID50 / 0.1mL.
Embodiment 2 6
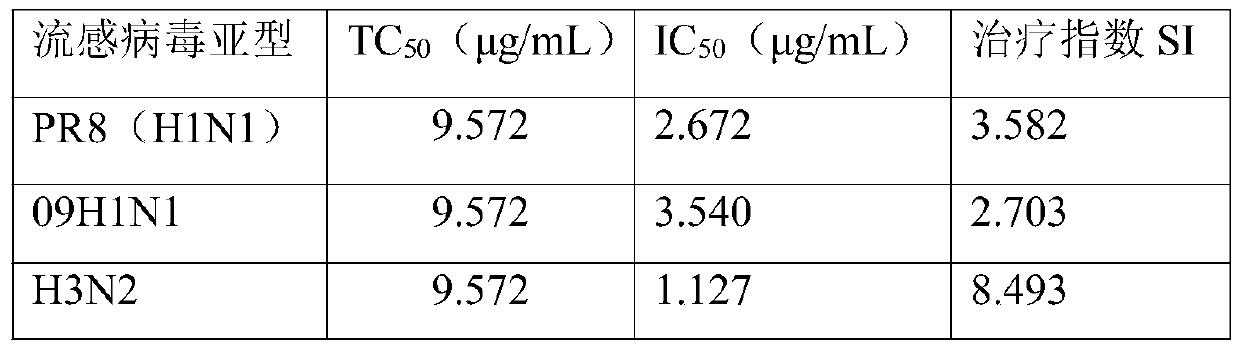
[0031] Example 2 Liuling Jiedu Pill Anti-influenza Virus Efficacy Evaluation
[0032] 1. Method.
[0033] The monolayer cells of 96-well plate were washed once with PBS, and 100 μL / well of influenza virus dilution solution containing about 100 TCID 50 was added, at 37°C, 5% CO 2 Incubate in the incubator for 2 hours, discard the virus solution, add 2-fold gradient dilution of Liuling Jiedu Pill drug, set 4 replicate wells for each concentration, take the maximum non-toxic concentration as the initial concentration of the drug, at 37°C, 5% CO 2 Incubate for 2 days in the incubator. Observe the cell lesion, stain with MTT, and measure the OD value. The half effective drug concentration (IC50) was calculated by Reed-Muench method, and the therapeutic index (SelectiveIndex, SI) was calculated: SI=TC50 / IC50). [Refer to "Pharmacological Experimental Methodology, edited by Xu Shuyun", the therapeutic index (SI)>1 means effective].
[0034] 2. Results.
[0035] The efficacy of L...
Embodiment 3
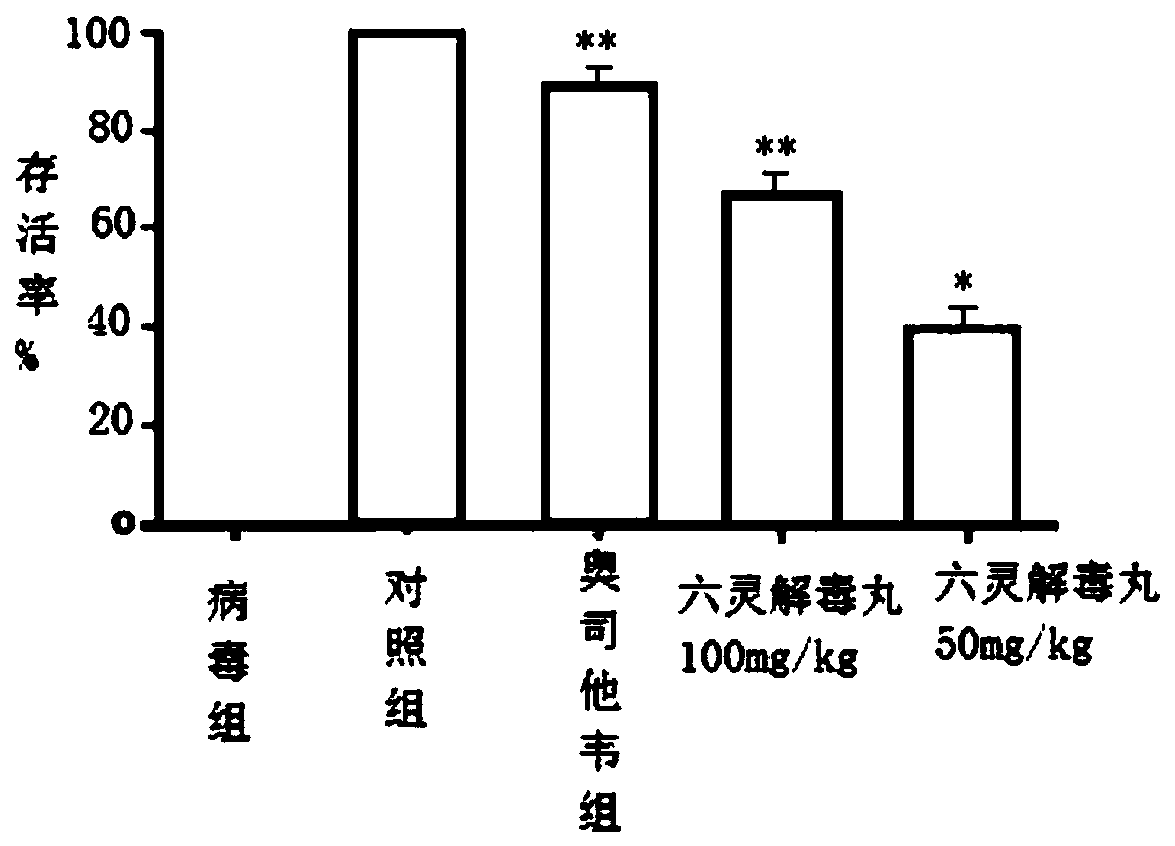
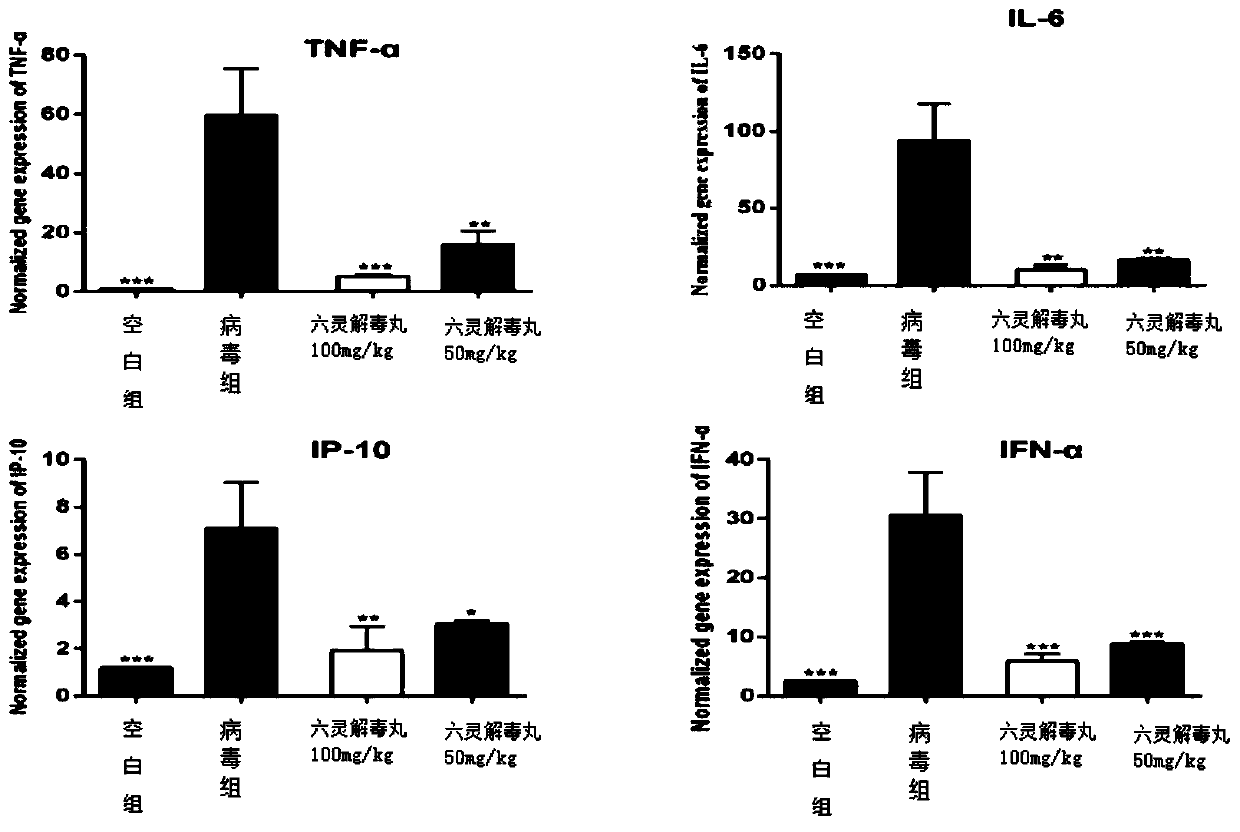
[0040] Evaluation of the protective effect of Liuling Jiedu Pill against influenza virus death in vivo.
[0041] 1. Method.
[0042] Female BALB / c mice were randomly divided into groups according to body weight. Except for the normal control group, the mice in other groups were anesthetized with ether, and the mice were given nasal drops with 2LD50 PR8 subtype virus liquid, 50 μL / mouse. The normal control group was given intranasal drops of the same volume of normal saline. The administration was started 2 hours after the intranasal infection of the virus, and the administration was continued for 5 days. Observations started from the first day of virus infection and continued for 14 days. The mental state, diet, respiration, coat color, body weight changes and death of the infected mice were observed and recorded every day.
[0043] 2. Results.
[0044] Death protection results such as figure 1 As shown, NC in the figure is the normal control group, the oseltamivir group (...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com