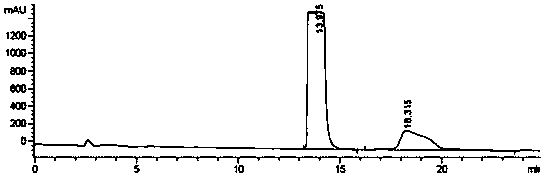
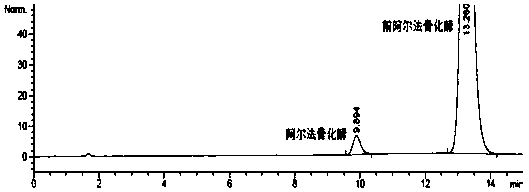
Alfacalcidol precursor preparation method
A technology of alphacalcidol and alfacalcidol, which is applied in the direction of organic chemistry, can solve the problems of large consumption of raw materials, long development cycle, high material cost, etc., and achieve low cost, improved utilization and simple preparation process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0020] A preparation method for pro-alphacalcidol, is characterized in that, comprises the following steps:
[0021] 1) The obtained alfacalcidol raw material is placed in a reactor (round bottom flask) and dissolved with absolute ethanol.
[0022] The raw material weight of alfacalcidol is 200 mg, and the volume of absolute ethanol is 100 mL.
[0023] 2) Heat the reactor described in step 1, heat it in a hot water bath at 80°C to condense and reflux for 2 hours.
[0024] 3) Take out the product in the reactor in step 2), and remove the ethanol by rotary evaporation under reduced pressure in a hot water bath at 60°C.
[0025] 4) Add mobile phase to dissolve the product obtained in step 3), that is, liquid phase preparation
[0026] The volume ratio of each component of the mobile phase is
[0027] 8 parts n-heptane
[0028] 9 parts ethyl acetate
[0029] 3 parts dichloromethane
[0030] The weight of the product obtained in step 3) is about 200mg, and the volume of the m...
Embodiment 2
[0035] A preparation method for pro-alphacalcidol, is characterized in that, comprises the following steps:
[0036] 1) The obtained alfacalcidol raw material is placed in a reactor (round bottom flask) and dissolved with absolute ethanol.
[0037] The raw material weight of alfacalcidol is 400mg, and the volume of absolute ethanol is 100mL.
[0038] 2) Heat the reactor described in step 1, heat in a hot water bath at 90°C, condense and reflux for 1.5 hours.
[0039] 3) Take out the product in the reactor in step 2), and remove the ethanol by rotary evaporation under reduced pressure in a hot water bath at 60°C.
[0040] 4) Add mobile phase to dissolve the product obtained in step 3), that is, liquid phase preparation
[0041] The volume ratio of each component of the mobile phase is
[0042] 8 parts n-heptane
[0043] 9 parts ethyl acetate
[0044] 3 parts dichloromethane
[0045] The weight of the product obtained in the step 3) is about 400mg, and the volume of the mo...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com