Synthetic method for rebaudioside R
A technology of stevioside and compound, which is applied in the field of synthesis of stevioside R, can solve problems such as methods to be optimized, and achieve the effects of ensuring synthesis efficiency, increasing synthesis efficiency, and reducing synthesis steps
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0056] The preparation method of some compounds in the present invention is as follows:
[0057] (1) Preparation method of compound 1
[0058]
[0059] Starting from glucose, compound 1 was obtained with a yield of 70% after three steps of reaction. .
[0060] (2) Preparation method of compound 2
[0061]
[0062] Compound 11 was prepared by referring to the method in J.Carbohydr.Chem.2007, 26, 61-82, and the 4-position hydroxyl group was dissolved in compound 11 (5.9g, 19.9mmol) in anhydrous DMF (25mL), and added in batches at 0°C NaH (0.96 g, 40.0 mmol). N 2 After stirring for 20 minutes under the atmosphere, NapBr (5.27 g, 23.8 mmol) was added to the reaction mixture, and stirred at this temperature for 3 hours. The reaction was quenched with water, diluted with ethyl acetate, washed with water and brine, then washed with anhydrous Na 2 SO 4 Drying, filtration, and concentration under reduced pressure gave a crude product, which was further purified by silica g...
Embodiment 1
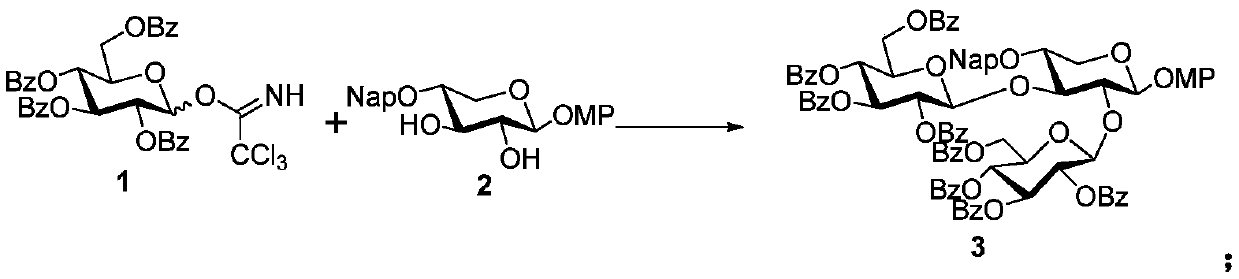
[0070] Step 1: 4-Methoxyphenyl-2,3-di-O-(2,3,4,6-tetra-O-benzoyl-β-D-glucopyranosyl)-4-O -(2-Naphthylmethyl)-β-D-xylopyranoside (3)
[0071] To the anhydrous CH of 1 (280mg, 0.38mmol) and 2 (50mg, 0.126mmol) 2 Cl 2 (1 mL) activated powdered 4A molecular sieves (80 mg) were added to the solution. The resulting suspension was stirred at the same temperature for 30 minutes, then cooled to -40°C, and TMSOTf (14.4 μL, 0.08 mmol) was added thereto. After the reaction solution was stirred at -40°C for another 1 hour, Et 3 N to quench the reaction. After filtration, it was concentrated under reduced pressure, and the resulting residue was purified by silica gel column chromatography to obtain Compound 3 (150 mg, 77%) as a colorless foam. [α] D 25 =+70.8(c 0.5, CHCl 3 ); 1 H NMR (400MHz, CDCl 3 )δ8.33(d, J=7.2Hz, 2H), 8.26(d, J=7.2Hz, 2H), 7.98-7.28(m, 47H), 6.85(d, J=9.2 Hz, 2H), 6.58( d, J=8.8Hz, 2H), 5.87(q, J=9.6Hz, 2H), 5.66-5.53(m, 4H), 5.01(d, J=12.4Hz, 1H), 4.92(d, J=8...
Embodiment 2
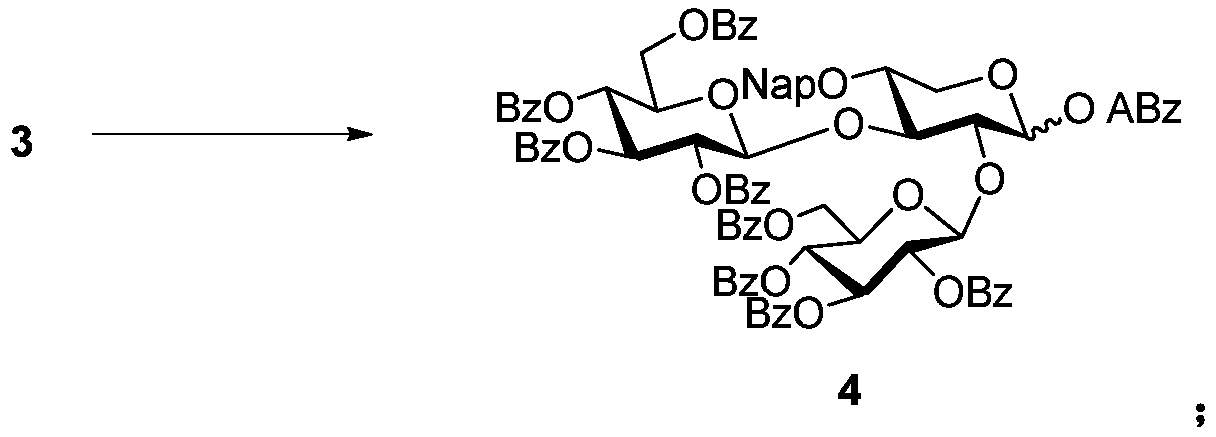
[0083] The method preparation of reference example. The difference is:
[0084] In step (1), the mol ratio of Compound 1 and Compound 2 is 1:1, and the mol ratio of TMSOTf and Compound 2 is 0.7:1;
[0085] In step (2a), the volume ratio of acetonitrile and the buffer solution is 1:1, the molar ratio of CAN and compound 3 is 5:1, and the concentration of compound 3 in the mixed solution is 0.1mol / L;
[0086] In step (2b), the hemiacetal intermediate, ABzOH, iPr 2 The molar ratio of NEt, DMAP and EDCI is 1:1.5:4:2:4;
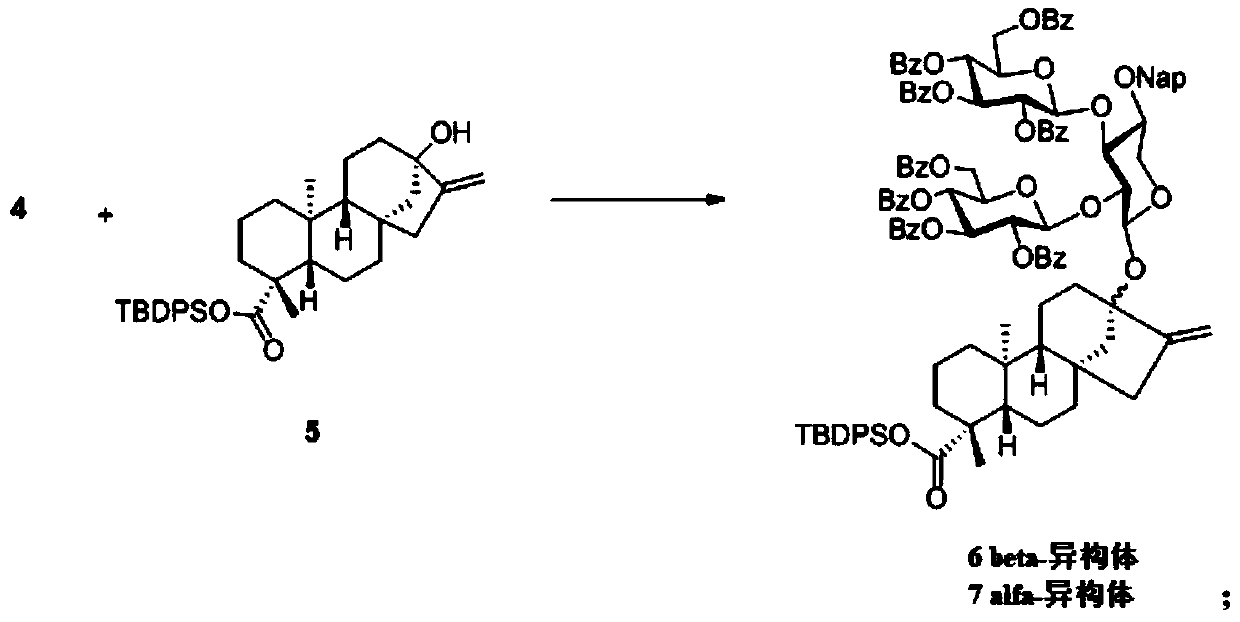
[0087] In step (3), Ph 3 wxya 2 The molar ratio of compound 4 and compound 4 is 0.1:1, and the molar ratio of compound 4 and compound 5 is 1:1; the concentration of compound 5 in acetonitrile is 0.01mol / L.
[0088] In step (4), the molar ratio of compound 6, HOAc and TBAF is 1:4:2; the concentration of compound 6 in the solvent is 0.02mol / L;
[0089] In step (5), the volume ratio of chloroform and water is 1:1.5, compound 8, K 2 CO 3 , TBAB and the molar r...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com