Drug composition used for treating MRSA and applications thereof
A composition and drug technology, applied in the field of medicine, can solve problems such as difficulty in treating infection, reducing membrane permeability, and changing the target of antibiotics
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment
[0025] tablet
[0026] Compound A 20g talcum powder 10g
[0027] Compound B 12g micronized silica gel 1g
[0028] Microcrystalline Cellulose 25g Magnesium Stearate 1g
[0029] Starch 23g
[0030] A total of 1000 tablets were prepared
[0031] Weigh the prescribed amount of compound A and compound B, fully mix them in a mortar, pass through an 80-mesh sieve, then mix with other excipients in the prescription, pass through a 40-mesh sieve, and directly compress the obtained powder into tablets.
experiment example 1
[0032] In vitro evaluation of the bacteriostatic effect of experimental example 1 pharmaceutical composition of the present invention on MRSA
[0033] The purpose of this experiment is to investigate the in vitro inhibitory effect of the pharmaceutical composition of the present invention on MRSA using the minimum inhibitory concentration (MIC) as an index.
[0034] 1. Experimental materials
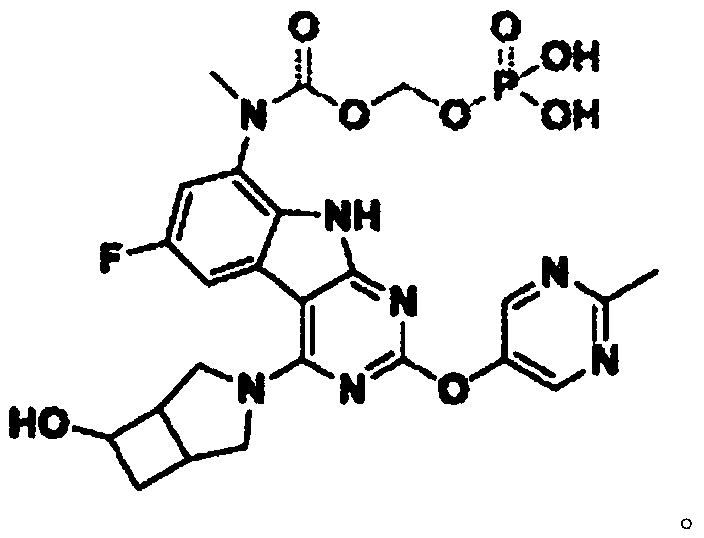
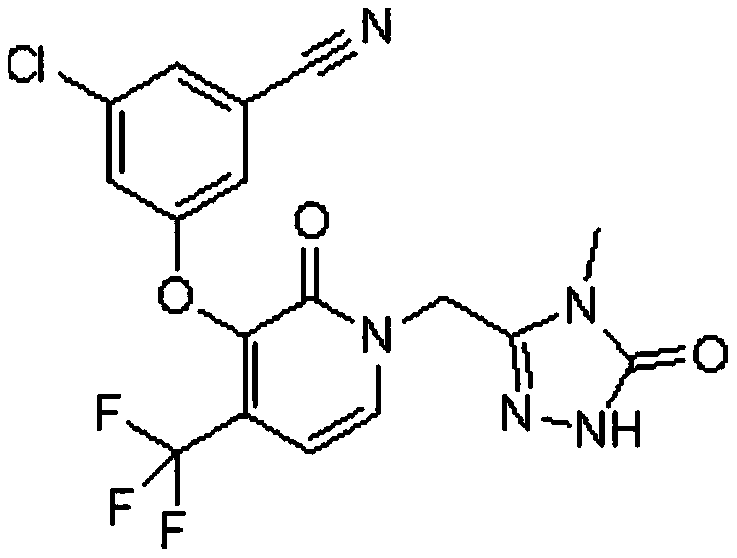
[0035] Resazurin was purchased from Shanghai Hualan Chemical Technology Co., Ltd.; Compound A and Compound B were synthesized according to the methods disclosed in patent documents WO2015 / 038661A1 and WO 2011 / 120133A1 respectively; MRSA clinical isolates were obtained from Clinical Pharmacology Research of Peking University Institute; Corning359996-well cell culture plate, purchased from Corning (Corning) Company.
[0036] 2. Experimental method
[0037] In this experiment, the resazurin chromogenic method was used to determine the minimum inhibitory concentration (MIC) of samples agai...
experiment example 2
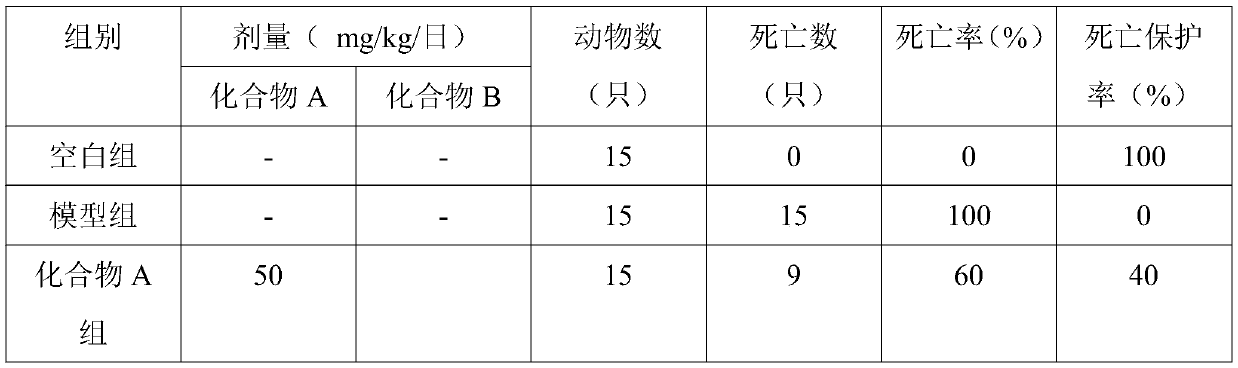
[0048] Experimental Example 2 Effect of the pharmaceutical composition of the present invention on the death protection rate of mice infected with MRSA
[0049] The purpose of this experiment is to investigate the in vivo therapeutic effect of the pharmaceutical composition of the present invention on MRSA-infected mice.
[0050] 1. Experimental materials
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com