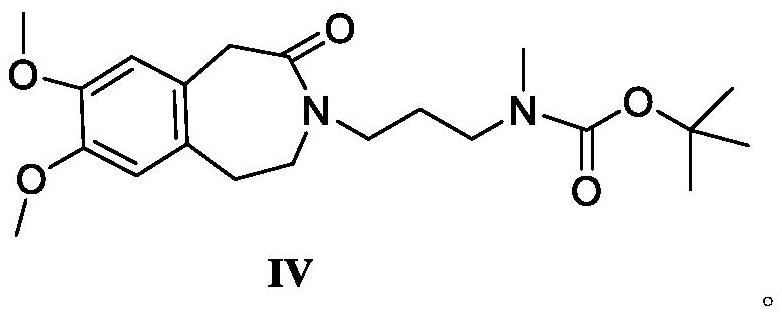
Ivabradine intermediate compound IV
A technology for ivabradine and compounds, applied in the field of ivabradine intermediate compound IV, can solve the problems of high technical requirements, high production costs, environmental pollution, etc., achieve economical and environmentally friendly yields, easy operation, and high product purity Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
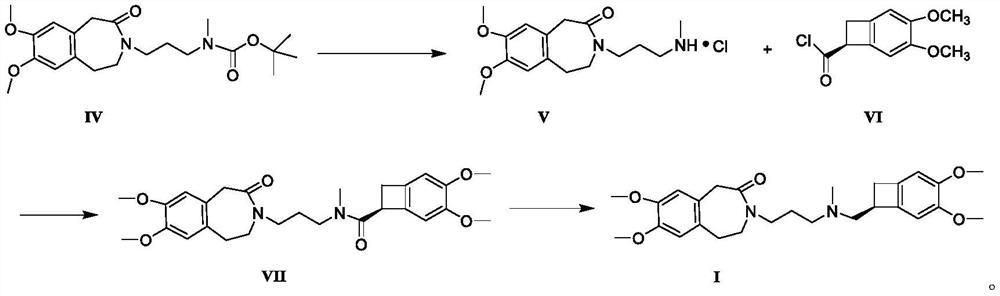
Image
Examples
Embodiment 1
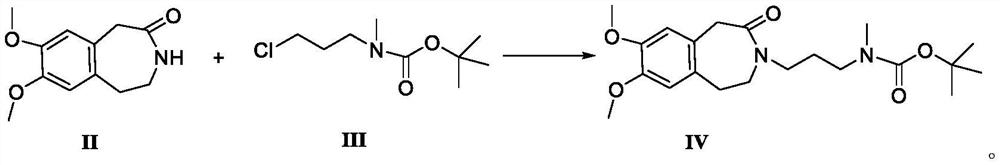
[0055] Compound II, 7,8-dimethoxy-1,3,4,5-tetrahydro-benzazepine-2-one (44.3g, 0.2mol) was dissolved in 250ml DMSO at room temperature, and added in batches Potassium tert-butoxide (26.9g, 0.24mol) was stirred for 10min, and compound III (3-chloro-propyl)-methyl-carbamic acid tert-butyl ester (53.9g, 0.26mol) and DMSO ( 120ml) mixed solution, after the reaction was completed, the reaction solution was poured into pure water (150ml), stirred to precipitate solid, suction filtered, added 200ml acetone for beating, suction filtered until no filtrate flowed out, and vacuum dried at 40°C to obtain compound IV. 99.8%, HPLC purity 99.85%.
Embodiment 2
[0057] Compound II, 7,8-dimethoxy-1,3,4,5-tetrahydro-benzazepine-2-one (44.3g, 0.2mol) was dissolved in 250ml DMSO at room temperature, and added in batches Sodium tert-butoxide (23.1g, 0.24mol) was stirred for 10min, and compound III (3-chloro-propyl)-methyl-carbamic acid tert-butyl ester (45.6g, 0.22mol) and DMSO ( 120ml) mixed solution, after the reaction was completed, the reaction solution was poured into pure water (150ml), stirred to precipitate solid, suction filtered, added 200ml acetone for beating, suction filtered until no filtrate flowed out, and vacuum dried at 40°C to obtain compound IV. 94.3%, HPLC purity 99.81%.
Embodiment 3
[0059] Compound II, 7,8-dimethoxy-1,3,4,5-tetrahydro-benzazepin-2-one (44.3g, 0.2mol) was dissolved in 250ml of benzene at room temperature, and added in batches Potassium carbonate (33.2g, 0.24mol) was stirred for 10min, and the mixed solution of compound III (3-chloro-propyl)-methyl-carbamic acid tert-butyl ester (82.9g, 0.40mol) and benzene (120ml) was added dropwise After the reaction was completed, the reaction solution was poured into pure water (150ml), stirred to precipitate solids, filtered with suction, added 200ml of acetone for beating, filtered with suction until no filtrate flowed out, and dried in vacuo at 40°C to obtain compound IV with a yield of 95.6% and an HPLC purity of 99.76 %.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com