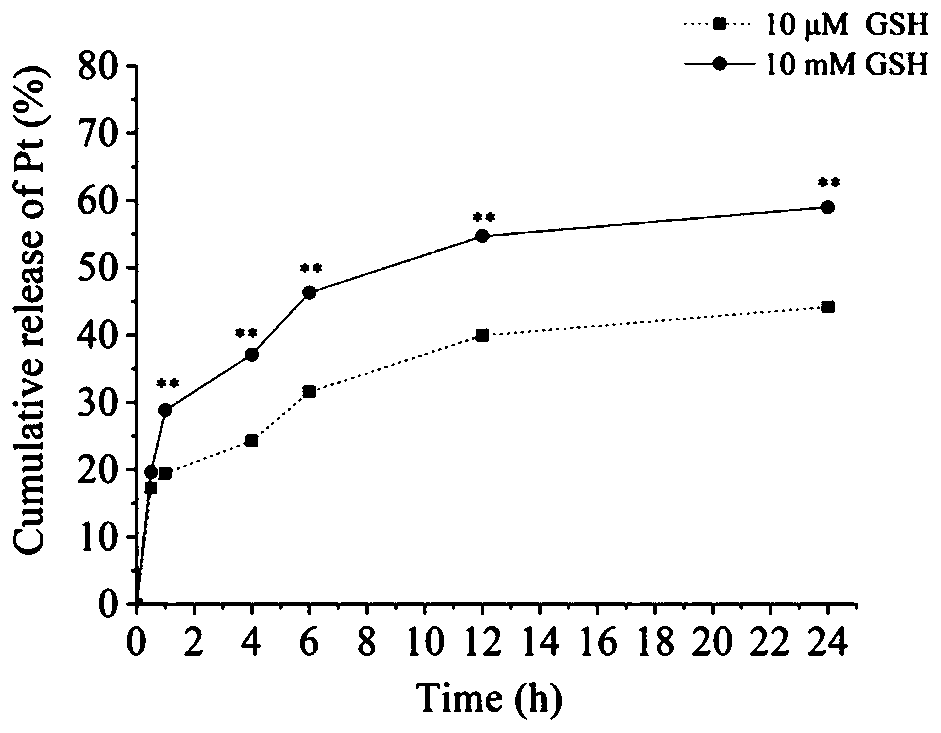
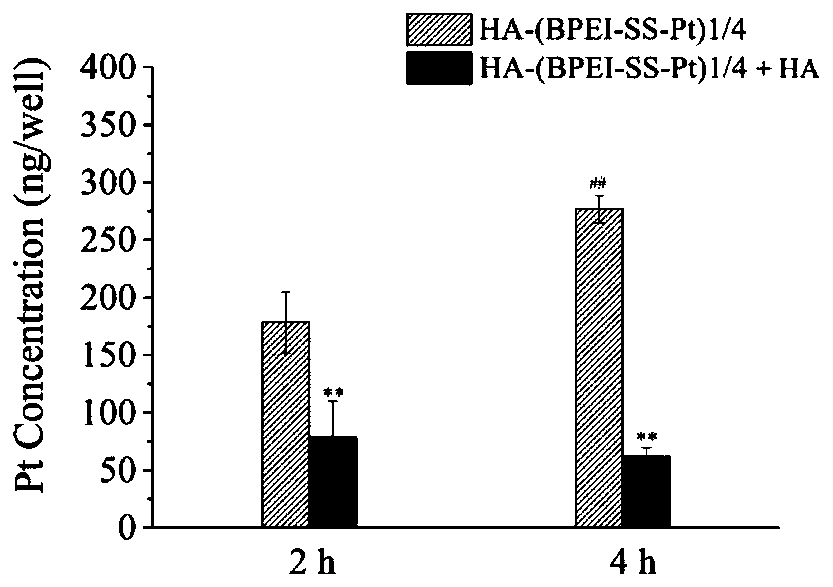
Preparation method of oxidoreduction responsiveness tumor-targeted cis-platinum nanometer drug delivery system, and application thereof
A nano-drug delivery system and tumor-targeting technology, which is applied in the preparation and application of redox-responsive tumor-targeted cisplatin nano-drug delivery system, can solve the problems of lack of specific ligands, large toxic and side effects, and effective drug utilization. Low rate and membrane permeability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0018] The preparation of embodiment 1 cisplatin complex
[0019] Weigh 675.0mg (2.23mmol) of cisplatin and add it into 225mL Grade III ultrapure water, stir at 37°C until completely dissolved, then cool to room temperature, add 758.2mg (4.45mmol) of silver nitrate, and continue to cool at room temperature The reaction was stirred for 48h. After the reaction, the reaction solution was centrifuged twice (5000 rpm, 1 h each time), and the supernatant was collected and filtered with a 0.1 μm water filter to obtain a hydrated cisplatin solution.
[0020] Cystamine dihydrochloride 499.4 mg (2.18 mmol) was dissolved in 24.6 mL of methanol at room temperature. Under ice-bath conditions, 445.7 mg (4.36 mmol) of triethylamine was added to the methanol solution of cystamine dihydrochloride and stirred for 30 min. Weigh 202.4 mg (1.98 mmol) of succinic anhydride and dissolve it in 36.97 mL of anhydrous 1,4-dioxane at room temperature, add the above solution to the methanol solution of ...
Embodiment 2
[0023] The preparation of embodiment 2 cisplatin polymer prodrug BPEI-SS-Pt
[0024] Weigh 48.8 mg (2 μmol) of branched polyethyleneimine (BPEI) and dissolve it in 5 mL of grade III ultrapure water, and ultrasonicate at 60°C for 15 minutes until completely dissolved to obtain a colorless and clear BPEI aqueous solution; cisplatin complex 77.3 mg (143 μmol) Dissolve in 10mL grade III ultrapure water, stir at room temperature until completely dissolved, and obtain a yellow, clear and transparent aqueous solution of cisplatin complex. Add 25.6 mg (158 μmol) of carbonyldiimidazole to the aqueous solution of the cisplatin complex, stir in an ice bath for 1 hour, then remove the ice bath. After the reaction solution returns to room temperature, add BPEI aqueous solution to the reaction solution, and stir at room temperature for 24 hours in the dark. After the reaction, the reaction solution was transferred to a dialysis bag with a molecular weight cut-off of 7000, dialyzed in 2000 m...
Embodiment 3
[0026] Example 3 Preparation of redox responsive tumor targeting cisplatin nano drug delivery system HA-(BPEI-SS-Pt)-1 / 4
[0027] Take the 1:4 mass ratio of HA and BPEI-SS-Pt as an example. Weigh 10.0mg of BPEI-SS-Pt and dissolve it in 10mL of grade III ultrapure water, and incubate at 50°C for 2 hours in the dark to dissolve completely, and obtain a concentration of 1mg·mL -1 BPEI-SS-Pt aqueous solution. Weigh 30.0mg HA and dissolve it in 6mL Grade III ultrapure water, shake until completely dissolved, and obtain a concentration of 5mg·mL -1 HA aqueous solution. Absorb the corresponding volume of HA solution according to the mass ratio of 1:4 (HA:BPEI-SS-Pt), add it to the aqueous solution of BPEI-SS-Pt, avoid light and sonicate at room temperature for 1h to obtain the composite nanoparticle HA-(BPEI-SS-Pt )-1 / 4, protected from light and stored in a refrigerator at 4°C. The particle size and potential of the nanoparticles were measured by a laser nanometer particle size a...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Molecular weight | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com