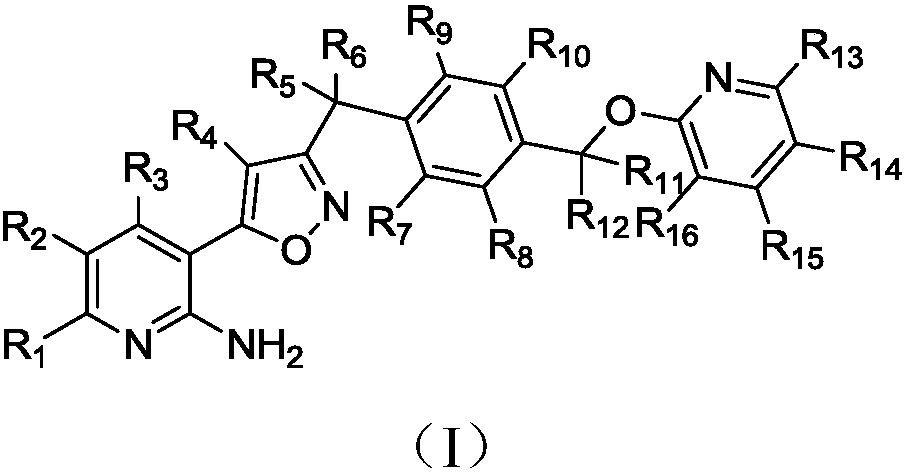
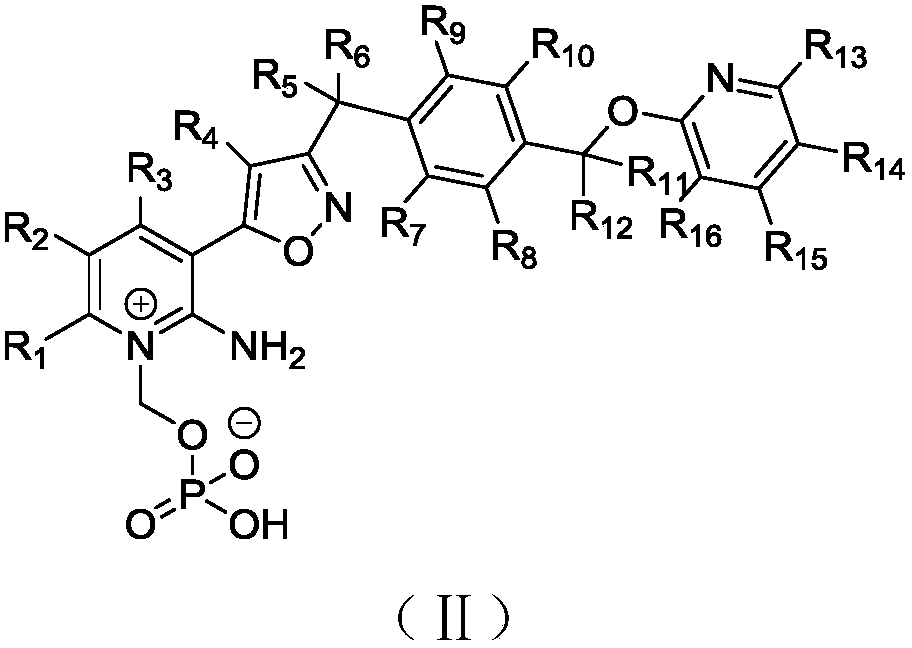
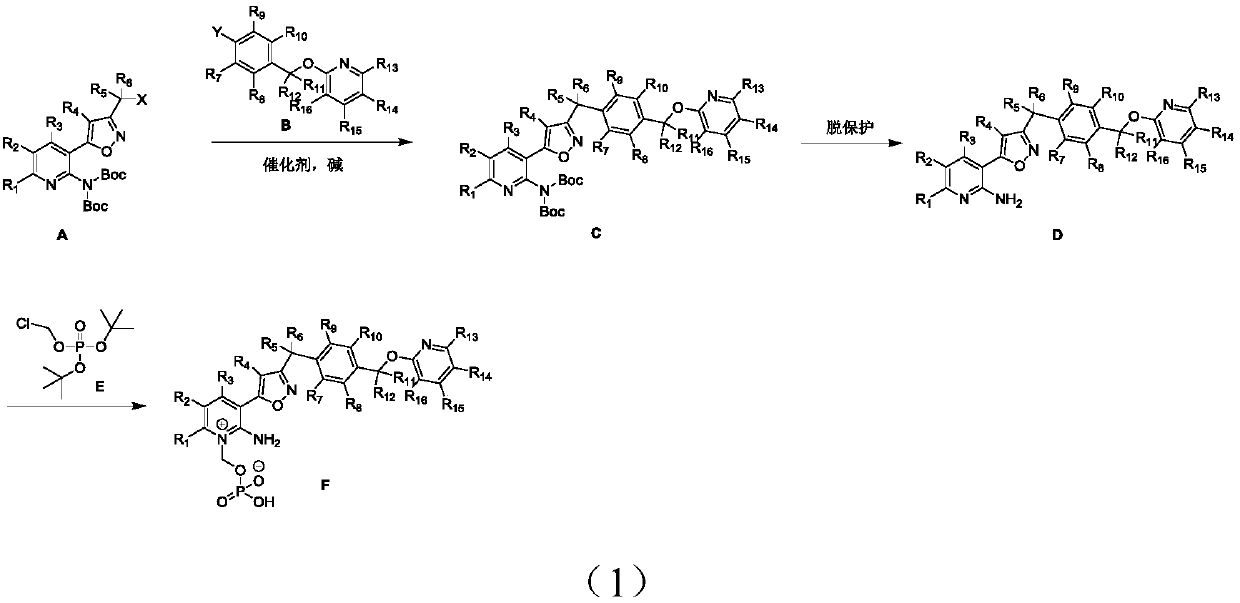
Preparation method and applications of deuterated isoxazole compound
A compound, the technology of isoxazole, applied in the field of medicinal chemistry, achieves good antifungal activity, improved stability, and improved water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0067] Synthesis of Compound D1:
[0068]
[0069] Step 1: Dissolve compound 1 (10mmol) in toluene, add pivaloyl chloride (15mmol) under ice bath, then slowly add triethylamine (16mmol) dropwise, react at room temperature until the raw materials are completely reacted, pour into water, stir and divide layer, and the aqueous phase was adjusted to pH 8-9 with 5N NaOH aqueous solution, then extracted with ethyl acetate, and the organic phases were combined, dried and spin-dried to obtain the crude product 2, which was directly used in the next step.
[0070] Step 2: Dissolve compound 2 (10 mmol) in absolute ethanol, add diethyl oxalate (15 mmol), dropwise add potassium tert-butoxide ethanol solution with a concentration of 22% under ice melting, add hydrochloric acid after the reaction is complete under ice melting Hydroxylamine (20mmol), add a small amount of water after the reaction is complete at room temperature, and then adjust the pH value to 6.5-7 with aqueous sodium hy...
Embodiment 2
[0079] Synthesis of compound D2:
[0080]
[0081] Step 1: Dissolve compound 1 (10 mmol) in anhydrous tetrahydrofuran, add triethylamine (5 mmol), add sodium borodeuteride (20 mmol) in batches under ice bath conditions, add deuterated methanol dropwise, and react at room temperature until complete Then add aqueous sodium hydroxide solution (50 mmol), heat to 60°C until the reaction is complete, adjust the pH value to 7-8 with hydrochloric acid, spin out the methanol, and carry out stirring and crystallization to obtain compound 2.
[0082] Step 2: Dissolve compound 2 (10mmol) in NMP, add thionyl chloride (20mmol), react at room temperature, add water after the reaction is complete, adjust the pH value to neutral with aqueous sodium hydroxide solution, and then use ethyl acetate After extraction, the organic phase was spin-dried to obtain compound 3.
[0083] Step 3: Dissolve compound 3 (10mmol) and 4-dimethylaminopyridine (1mmol) in ethyl acetate, add di-tert-butyl dicarbo...
Embodiment 3
[0086] Synthesis of compound D3:
[0087]
[0088] Step 1: Dissolve compound 1 (2.5g) in heavy water (100mL), add Pd / C (250mg), and react at 180°C for 24 hours in an autoclave or in a microwave reactor for 2 hours. After the reaction is complete, add Diluted with methanol, filtered off palladium carbon, spin-dried, and obtained compound 2 by column chromatography.
[0089] Step 2: Dissolve compound 2 (2 mmol) in 48% hydrobromic acid, add bromine and sodium nitrite aqueous solution under ice cooling, after the reaction is complete under ice cooling, add 30% sodium hydroxide aqueous solution to adjust the pH value, ethyl acetate After ester extraction, the organic phase was spin-dried and column chromatographed to obtain compound 3.
[0090] Step 3: Dissolve compound 4 (10mmol) in tetrahydrofuran, add 3 (12mmol), add potassium tert-butoxide (12mmol) under ice bath, react until complete at room temperature, pour into water, extract with ethyl acetate, dry and spin dry 5 late...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com