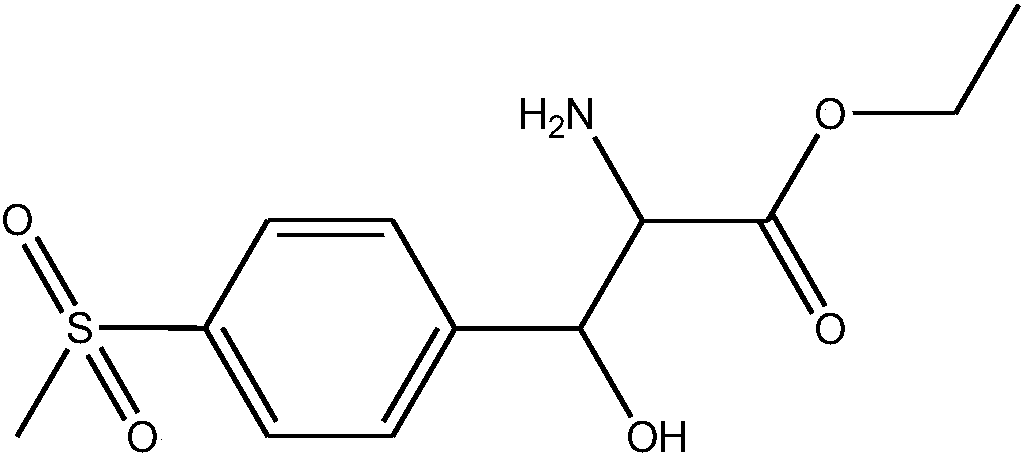
DL-p-methylsulfonylphenyl serine ethyl ester preparation method
A methylsulfonyl phenylserine ethyl ester, p-methylsulfonyl technology, applied in the field of preparation of DL-p-methylsulfonyl phenylserine ethyl ester, can solve the problems of low product yield, high cost, strong corrosiveness and the like, and achieves The effect of protecting the environment, avoiding danger and improving operational efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0021] 1) Add 83.7g (0.6mol) ethyl glycine hydrochloride into 500mL ethanol, heat to reflux, and dissolve ethyl glycine hydrochloride in ethanol;
[0022] 2) 66.3 (0.3mol) basic copper carbonate (Cu 2 (OH) 2 CO 3 ) was dissolved in 100mL ammonia water, and then added into the solution obtained in step 1);
[0023] 3) Add KOH to adjust the pH of the solution to 10.5 to 10.6 in step 2), add 92g (0.5mol) p-thiamphenicol benzaldehyde to react, keep the temperature at 78 to 85°C during the reaction, and control the pH to 10.0 to 11.0; by HPLC Monitor the progress of the reaction; when the percentage of p-thiamphenicol benzaldehyde calculated by the area normalization method<1%, the reaction ends.
[0024] 4) After the reaction is over, recover the ethanol in the reacted substance, then add ammonia water to the residue, adjust the pH to 8.0-8.2, cool the solution to 0-5°C, filter, wash with ice water, and dry to obtain 140.8g (0.49mol) p-thymphenylphenylserine ethyl ester, yield...
Embodiment 2
[0028] 1) Add 90.6g (0.65mol) ethyl glycine hydrochloride into 500mL ethanol, heat to reflux, and dissolve ethyl glycine hydrochloride in ethanol;
[0029] 2) 77.4 (0.35mol) basic copper carbonate (Cu 2 (OH) 2 CO 3 ) was dissolved in 100mL ammonia water, and then added into the solution obtained in step 1);
[0030] 3) In step 2), add KOH to adjust the pH of the solution=10.5~10.6, add 92g (0.5mol) p-thiamphenicol benzaldehyde for reaction, keep warm at 78~82°C during the reaction, control pH=10.0~11.0; by HPLC Monitor the progress of the reaction; when the percentage of p-thiamphenicol benzaldehyde calculated by the area normalization method<1%, the reaction ends.
[0031] 4) After the reaction is over, recover the ethanol in the reacted substance, then add ammonia water to the residue, adjust the pH=8.0-8.2, cool the solution to 0-5°C, filter, wash with ice water, and dry to obtain 141.4g (0.492mol) p-thymphenylphenylserine ethyl ester, yield: 98.4%, product purity 99.3%...
Embodiment 3
[0034] 1) Add 97.6g (0.7mol) ethyl glycine hydrochloride into 500mL ethanol, heat to reflux, and dissolve ethyl glycine hydrochloride in ethanol;
[0035] 2) 71.9 (0.325mol) basic copper carbonate (Cu2(OH)2CO3) was dissolved in 100mL ammonia water, and then added to the solution obtained in step 1);
[0036] 3) In step 2), add KOH to adjust the pH of the solution=10.5~10.6, add 92g (0.5mol) p-thiamphenicol benzaldehyde for reaction, keep warm at 78~82°C during the reaction, control pH=10.0~11.0; by HPLC Monitor the progress of the reaction; when the percentage of p-thiamphenicol benzaldehyde calculated by the area normalization method<1%, the reaction ends.
[0037] 4) After the reaction is over, recover the ethanol in the reacted substance, then add ammonia water to the residue, adjust the pH=8.0-8.2, cool the solution to 0-5°C, filter, wash with ice water, and dry to obtain 141.7g (0.493mol) p-thymphenylphenylserine ethyl ester, yield: 98.6%, product purity 99.4%.
[0038]...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com