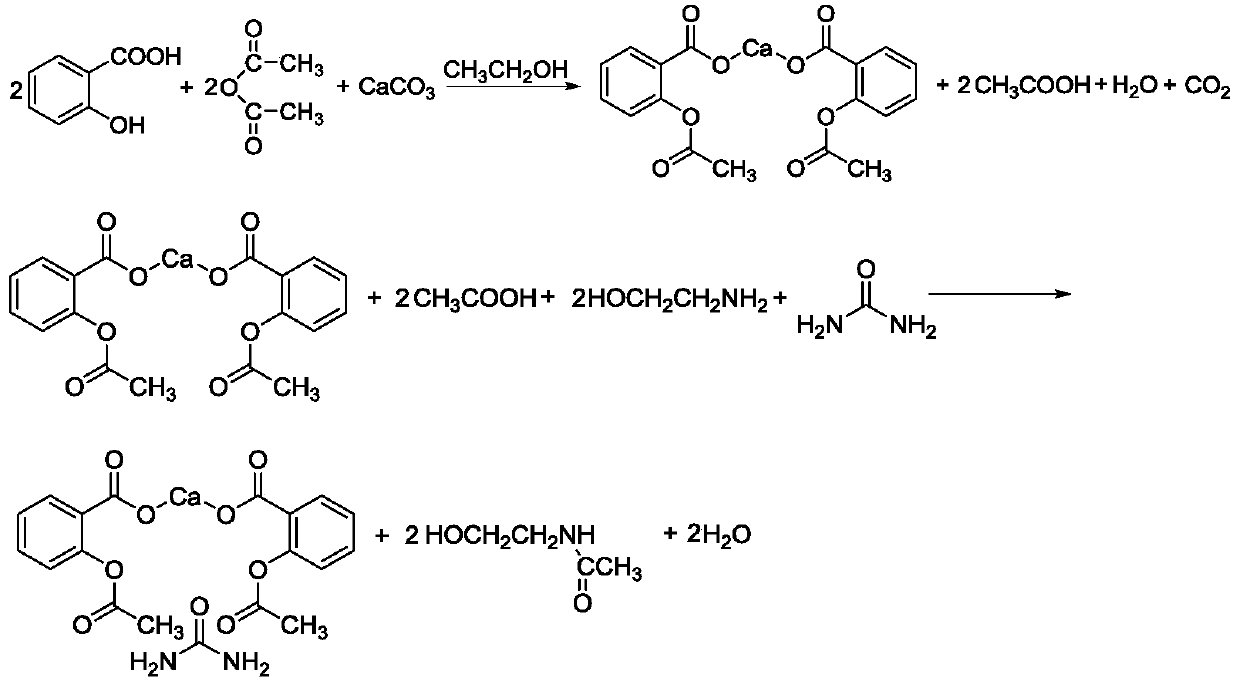
Synthesis process of carbaspirin calcium
A technology of carbasalate calcium and synthesis process is applied in the synthesis process of carbasalate calcium and in the field of preparation of organic medicines, which can solve the problems of high price and high production cost, reduce the cost of raw materials, ensure quality, and simplify the production process. Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
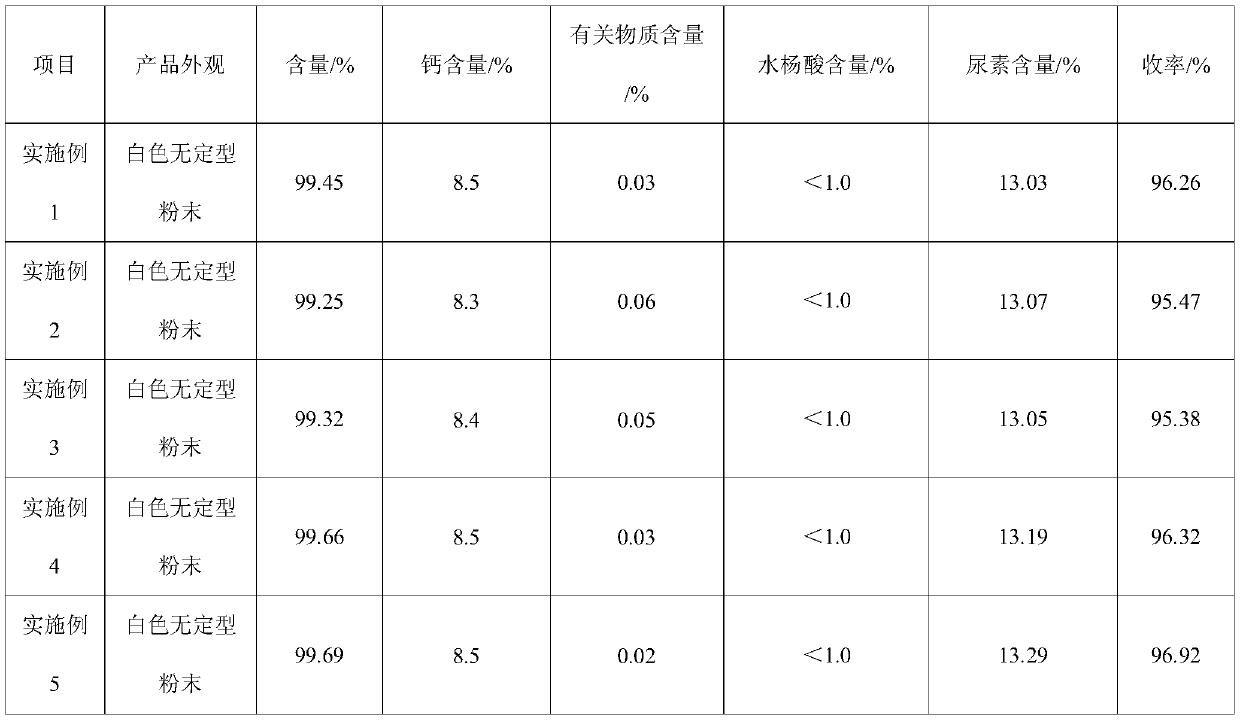
[0031] Add ethanol 180g, salicylic acid 276.24g (2mol), acetic anhydride 204.18g (2mol) successively in the 2L there-necked flask with reflux condenser and stirring, start stirring, add calcium carbonate 100.09g (1mol) in batches ( Divide the calcium carbonate into portions, add once every 10 minutes, and the amount added each time is 1.5 times the amount added before, and the addition is completed within 2 hours. The added amounts are 0.26g, 0.39g, 0.58g, 0.87g, 1.3 g..., until the addition is complete), the temperature is raised to 60°C, kept for 0.5h, and then cooled to 25°C. Then add 122.16g (2mol) of ethanolamine, 330.33g of pure water, and 66.07g of urea (1.1mol, the mass ratio of water to urea is 10:2), and the reaction time is 1h. After the reaction, cool down to 15°C, centrifuge, and rinse with 50g of ethanol , drying to obtain high-quality carbasalate calcium.
Embodiment 2
[0033] Add 180g of ethanol, 276.24g of salicylic acid, and 204.18g of acetic anhydride in sequence in a 2L three-necked flask with reflux condenser and stirring, start stirring, and add 100.09g of calcium carbonate in batches (calcium carbonate is divided into portions, every 15min Add once, and the amount added each time is 1.5 times the amount added before, and the addition is completed within 2.5 hours), the temperature is raised to 65°C, kept for 1h, and the temperature is lowered to 30°C. Then add 122.16g of ethanolamine, 330.33g of pure water, and 66.07g of urea (the mass ratio of water to urea is 10:2), and the reaction time is 2h. After the reaction, the temperature is lowered to 20°C, centrifuged, rinsed with 50g of ethanol, and dried to obtain high-quality carbasalate calcium.
Embodiment 3
[0035] Add 180g of ethanol, 276.24g of salicylic acid, and 204.18g of acetic anhydride in sequence in a 2L three-neck flask with reflux condenser and stirring, start stirring, and add 100.09g of calcium carbonate in batches (calcium carbonate is divided into portions, every 20min Add once, and the amount added each time is 1.5 times the amount added before, and the addition is completed within 3 hours), the temperature is raised to 60°C, kept for 0.5h, and the temperature is lowered to 30°C. Then add 122.16 g of ethanolamine, 360 g of pure water, and 66.07 g of urea. The reaction time is 3 hours. After the reaction, the temperature is lowered to 15° C., centrifuged, rinsed with 50 g of ethanol, and dried to obtain high-quality carbasalate calcium.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com