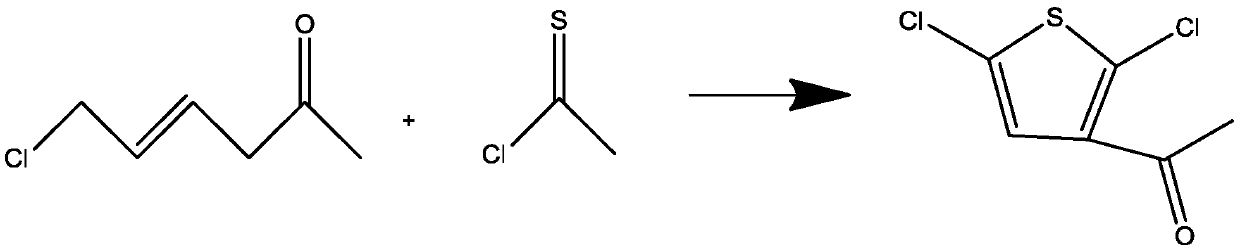
Synthesis method of 3-acetyl-2,5-dichlorothiophene
A technology of dichlorothiophene and synthesis method, which is applied in the direction of organic chemistry, can solve the problems of high production cost, large amount of waste water, poor product purity, etc., and achieve the effect of reducing requirements and reducing investment
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0033] Add 40g of 1-methyl-4-chlorobutenal to 200g of ethanol, stir and dissolve in a 500ml reaction bottle at a speed of 80 rpm, cool the reaction solution to -13°C, dissolve completely to form a solution, and add in batches Sodium ethylate 80g, feeding time 1.0h, after the feeding is finished, the temperature is raised to 0°C for 0.5h, after the reaction is finished, 80g of thioacetyl chloride solution is added dropwise to the reaction solution, and the temperature is raised to 60°C after the 1h dropwise addition process is completed, After reacting for 2 hours, distill ethanol off under reduced pressure, add 20 g of water to wash, separate the liquid, carry out rectification under reduced pressure on the organic phase, take 80-90 ° C fractions, and obtain 43.5 g of colorless transparent oily substance, the conversion rate is 75% , The gas phase content is 99.3%.
Embodiment 2
[0035] Add 100g of 1-methyl-4-chlorobutenal to 1000g of methanol, stir and dissolve in a 2500ml reaction bottle at a speed of 80 rpm, cool the reaction solution to -10°C, completely dissolve to form a solution, and add in batches Sodium methoxide 75g, the feeding time is 0.75h, after the feeding is finished, the temperature is raised to 2 degrees Celsius for 1 hour, after the reaction is finished, 60g of thioacetyl chloride solution is added dropwise to the reaction solution, and the temperature is raised to 80 degrees Celsius after 1.5 hours of dropping the process. After reacting for 1 hour, methanol was distilled off under reduced pressure, then 100 g of water was added to wash, the liquid was separated, and the organic phase was rectified under reduced pressure, and a fraction at 80-90 ° C was taken to obtain 108.9 g of a colorless transparent oil, with a conversion rate of 75.08% , The gas phase content is 99.4%.
Embodiment 3
[0037] Add 100g of 1-methyl-4-chlorobutenal to 600g of acetone, stir and dissolve in a 2000ml reaction bottle at a speed of 80 rpm, cool the reaction solution to -15°C, completely dissolve to form a solution, and add in batches Sodium bicarbonate 200g, the feeding time is 0.5h, after the feeding is finished, the temperature is raised to 10 degrees Celsius for 0.5 hours, after the reaction is finished, 70g of thioacetyl chloride solution is added dropwise to the reaction solution, and the temperature is raised to 75 degrees Celsius after 2 hours of the dropping process , reacted for 2 hours, after the acetone was distilled off under reduced pressure, 100 g of water was added to wash, and the liquid was separated, and the organic phase was subjected to vacuum distillation, and a fraction of 80-90 ° C was taken to obtain 117.6 g of a colorless transparent oil, with a conversion rate of 81.1 %, the gas phase content is 99.5%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com