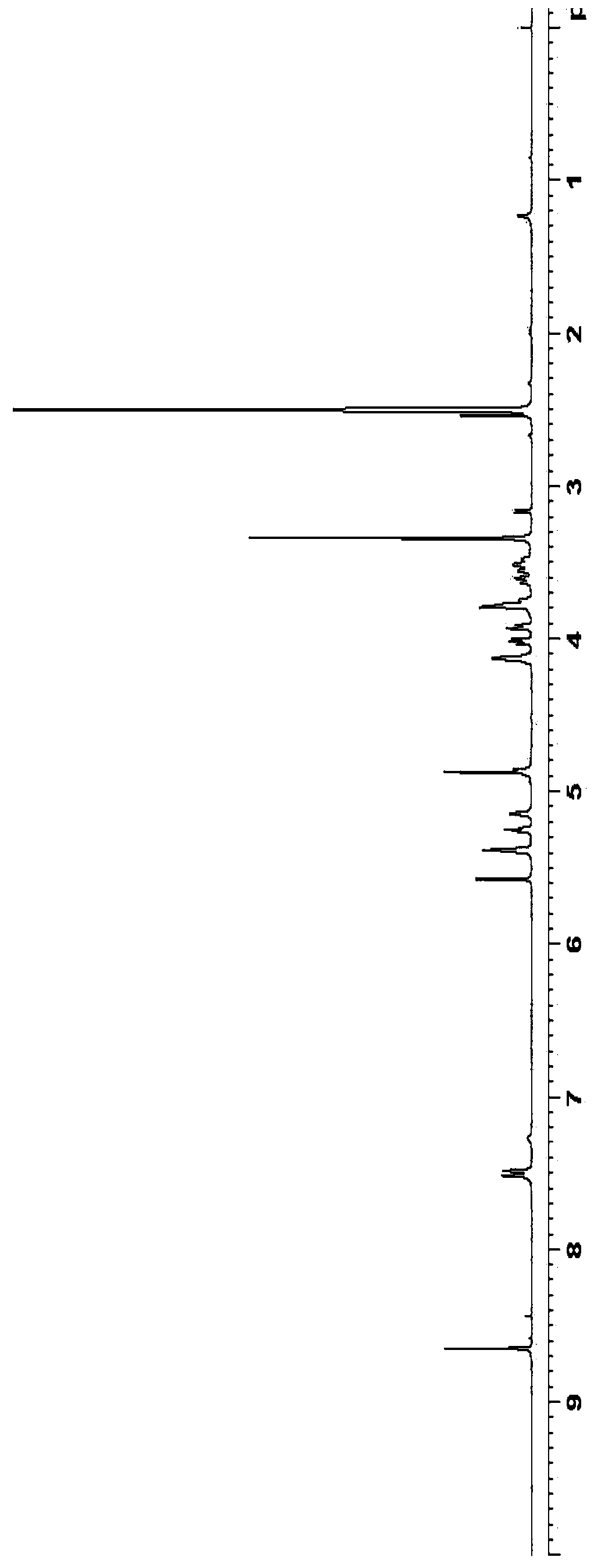
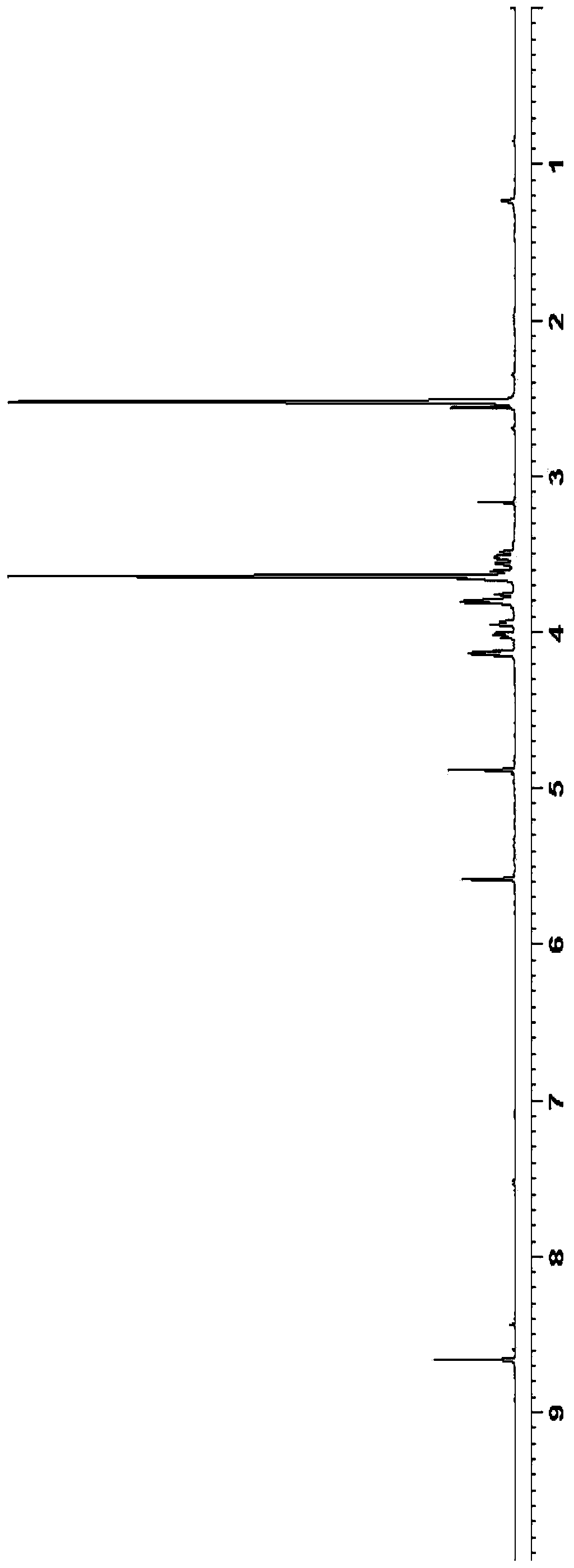
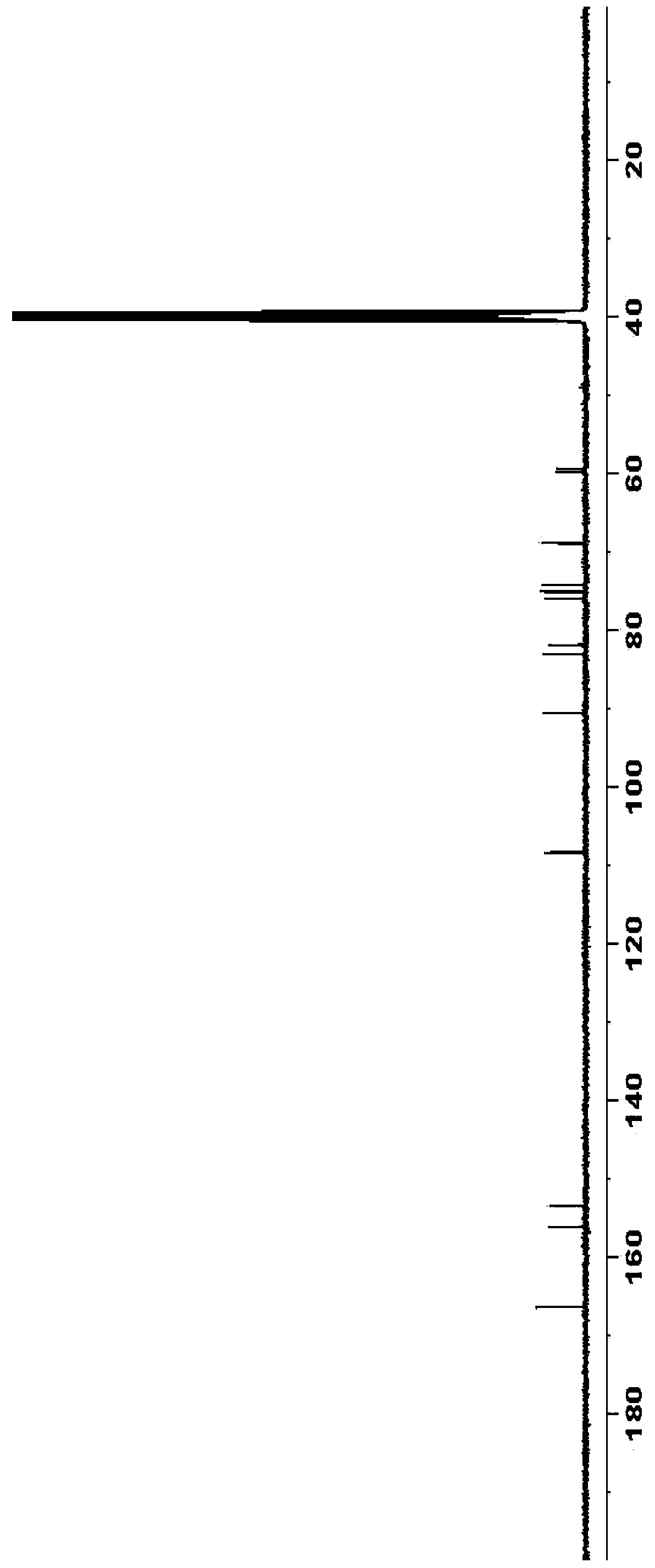
5-azacytidine compound and preparation method thereof
A technology of azacytidine nucleoside and compound, applied in the field of 5-azacytidine nucleoside compound related substances and its preparation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Preparation of silyl 5-azacytosine:
[0051]
[0052] 5-azacytosine (10.0 g), hexamethyldisilazane (200 mL) and ammonium sulfate (0.2 g) were mixed, heated to reflux, and the reaction was stirred for 18 hours. After the reaction was complete, it was concentrated under reduced pressure, heated with n-heptane (200 mL) to make a slurry, and filtered. The filter cake was dried at 50° C. for 12 hours to obtain 19.5 g of white silyl 5-azacytosine with a mass yield of 195% (calculated as 5-azacytosine).
Embodiment 2
[0054] Preparation of triacetyl azacitidine:
[0055]
[0056] Tetraacetylribose (38.3g) and silyl 5-azacytosine (19.5g) were added to dichloromethane (200mL), and at 20-30°C, trimethylsilyl trifluoromethanesulfonate (25.5 g) was added dropwise to the reaction solution. After the dropwise addition was completed, the reaction was stirred for 5 hours. After the reaction was completed, it was washed with sodium bicarbonate solution (100 mL), purified water (100 mL) and sodium chloride solution (100 mL), respectively. Add anhydrous magnesium sulfate to dry for 5 hours, filter, wash the filter cake with dichloromethane, and concentrate under reduced pressure to obtain 24.2 g of oily triacetylazacitidine, with a mass yield of 124% (based on silyl 5-azacitidine pyrimidine meter).
Embodiment 3
[0058] Preparation of diacetyl azacitidine:
[0059]
[0060] Triacetylazacitidine (24.2 g) was added to anhydrous methanol (250 mL), and a 30% methanol solution of sodium methoxide (6 mL) was added dropwise to the reaction solution at 0-10°C. After the dropwise addition was completed, the internal temperature of the reaction was controlled at 0-10° C. and the reaction was stirred for 12 hours. Acetic acid was added dropwise to adjust the pH to 6-7, concentrated, and purified by column chromatography to obtain 14.5 g of diacetyl azacitidine with a mass yield of 60% (calculated as triacetyl azacitidine).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com