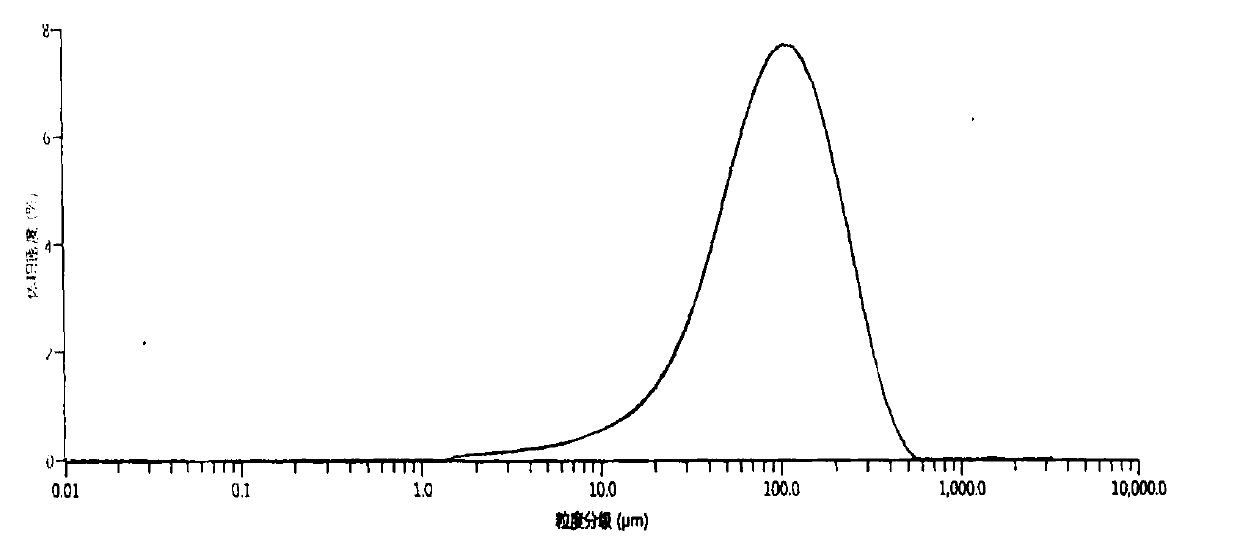
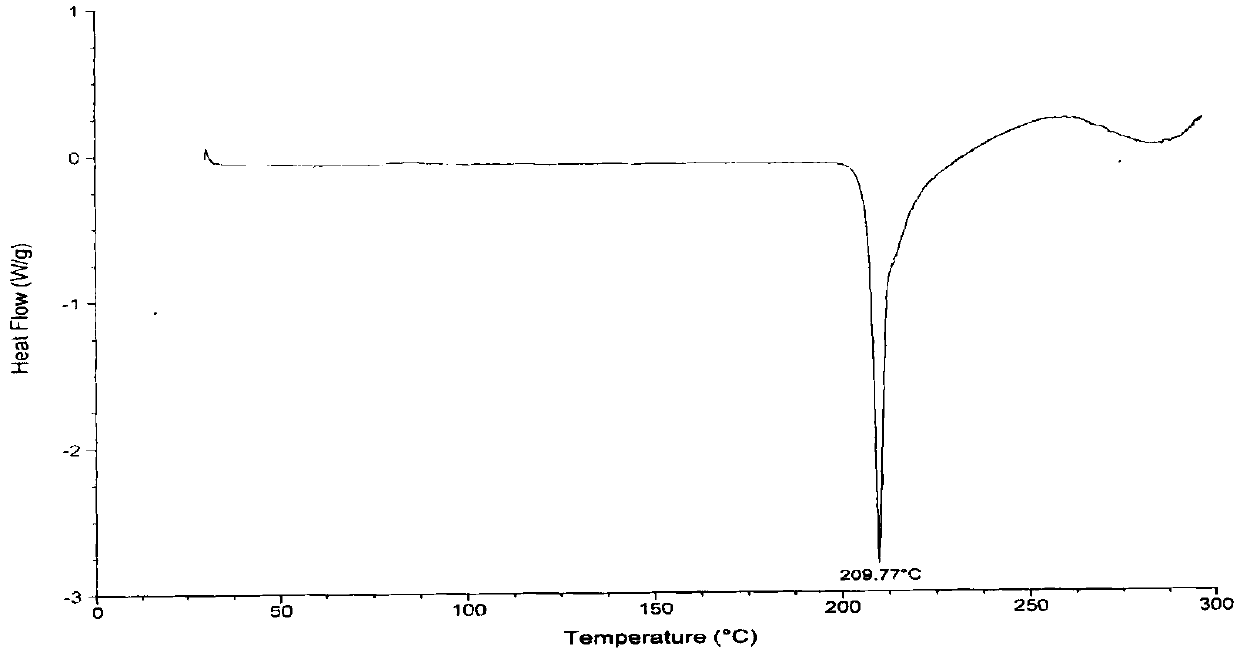
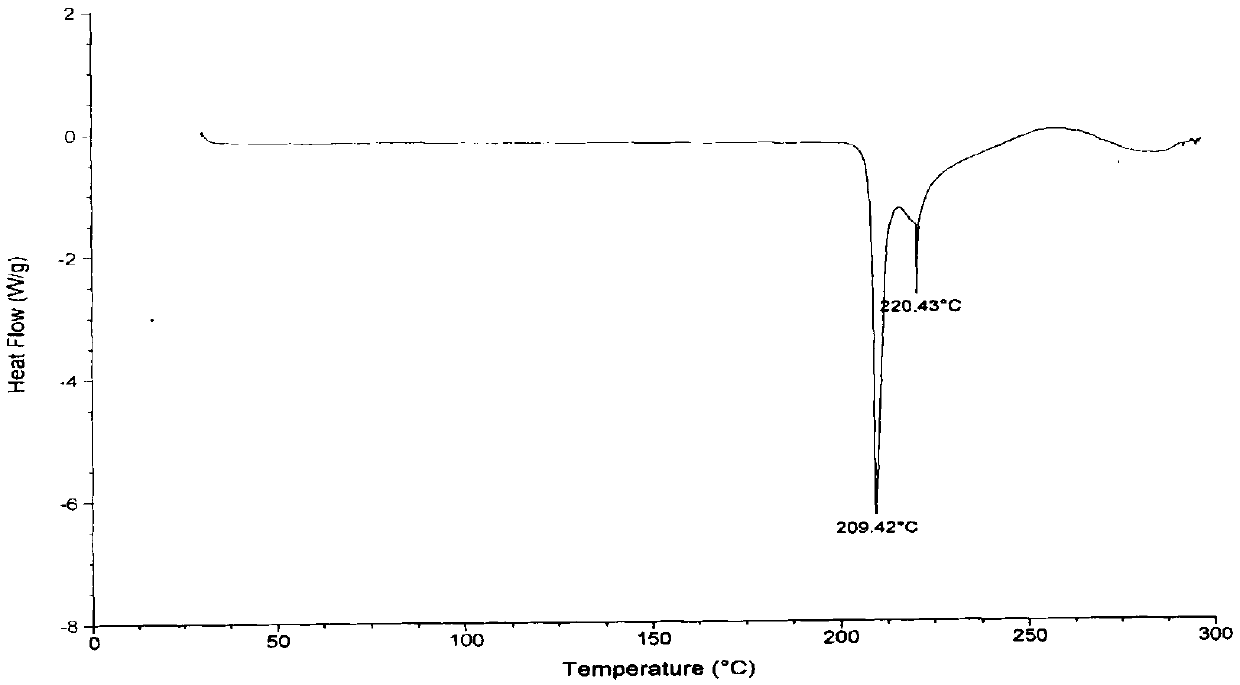
Vonoprazan fumarate preparation method
A technology of vonoprazan fumarate and oxalate, which is applied in the field of medicine, can solve the problems of coarse particle size, tedious refining steps, and low product purity of vonoprazan fumarate, and achieve reduced purification steps and high product quality. The effect of purity and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0040] Put methanol 1000ml, 5-(2-fluorophenyl)-1-(pyridin-3-ylsulfonyl)-1H-pyrrole-3-carbaldehyde 100.01g (0.303mol) into the reaction flask, add methylamine methanol solution 37.19g (0.359mol), stir, keep the inner temperature at 40±5°C and stir for 2h, cool down, keep the inner temperature at 0±5°C and add 5.73g (0.151mol) of sodium borohydride evenly in batches, continue to keep warm for 30min, add Quench the reaction with 200ml of water, continue to stir for 0.5h or more after dripping, distill methanol out under reduced pressure, add drinking water and 1000ml of ethyl acetate, stir, separate liquid, wash once with 200ml of water, put into the reaction bottle, add 150ml of ethanol, Stir, add 36.33g (0.288mol) of oxalic acid in portions, heat and reflux for 30min after addition, slowly cool down, crystallize at an internal temperature of 0-5°C for 1h, filter, drain, and dry the solid at 60-80°C for 3h to obtain oxalate Crude product 116.45g, yield 88.33%, purity 94.95%. In...
Embodiment 2
[0043] Put 40.06 g of the crude oxalate obtained in Example 1 into a reaction flask, add 320 ml of N,N-dimethylformamide (DMF), stir and heat, reflux to dissolve, cool down to crystallize, and the internal temperature is 0-5°C Crystallize for 1 h, filter, wash with 40 ml of ethanol, and dry to obtain 31.54 g of refined oxalate product 1 with a yield of 78.73% and a purity of 97.99%. In the related substance, impurity A is 0.67%, impurity B is 0.20%, impurity C is 0.24%, impurity D is 0.15%, and impurity 11 is 0.023%.
[0044] Put 10.48g of the above-mentioned solid oxalate refined product into the reaction tank, put 136ml of ethyl acetate and 14.51g of potassium carbonate into the reaction tank, stir and heat, dissociate for 3 hours at an internal temperature of 20±5°C, separate the liquid, and add sodium chloride Wash with 70ml of the solution, dry with 10.48g of anhydrous sodium sulfate, filter, wash with 10ml of ethyl acetate, put the filtrate into the reaction bottle, add ...
Embodiment 3
[0046] Put 10.03 g of the crude product of vonoprazan oxalate obtained in Example 1 into a reaction tank, add 71 ml of ethanol and 9 ml of water, stir and heat, reflux for 30 min, cool down and crystallize, crystallize at an internal temperature of 0-5 ° C for 1 h, filter , washed with 10ml of ethyl acetate, sucked dry, dried the obtained solid product at 60-80°C for 2.5h, and weighed to obtain the refined product of vonoprazan oxalate.
[0047] Put the refined product of Vonorazan oxalate, 110ml of ethyl acetate, 12.93g of potassium carbonate, and 60ml of water into the reaction tank, stir and heat, dissociate for 2 hours at an internal temperature of 20±5°C, separate the liquid, and wash with 3.3g of sodium chloride solution. Dry over anhydrous sodium sulfate, filter, wash with ethyl acetate, put the filtrate into the reaction bottle, add fumaric acid 2.49g methanol 40ml solution dropwise at 20±5°C, continue to react for 1h after dropping, lower the temperature until the inte...
PUM
| Property | Measurement | Unit |
|---|---|---|
| Particle size | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com