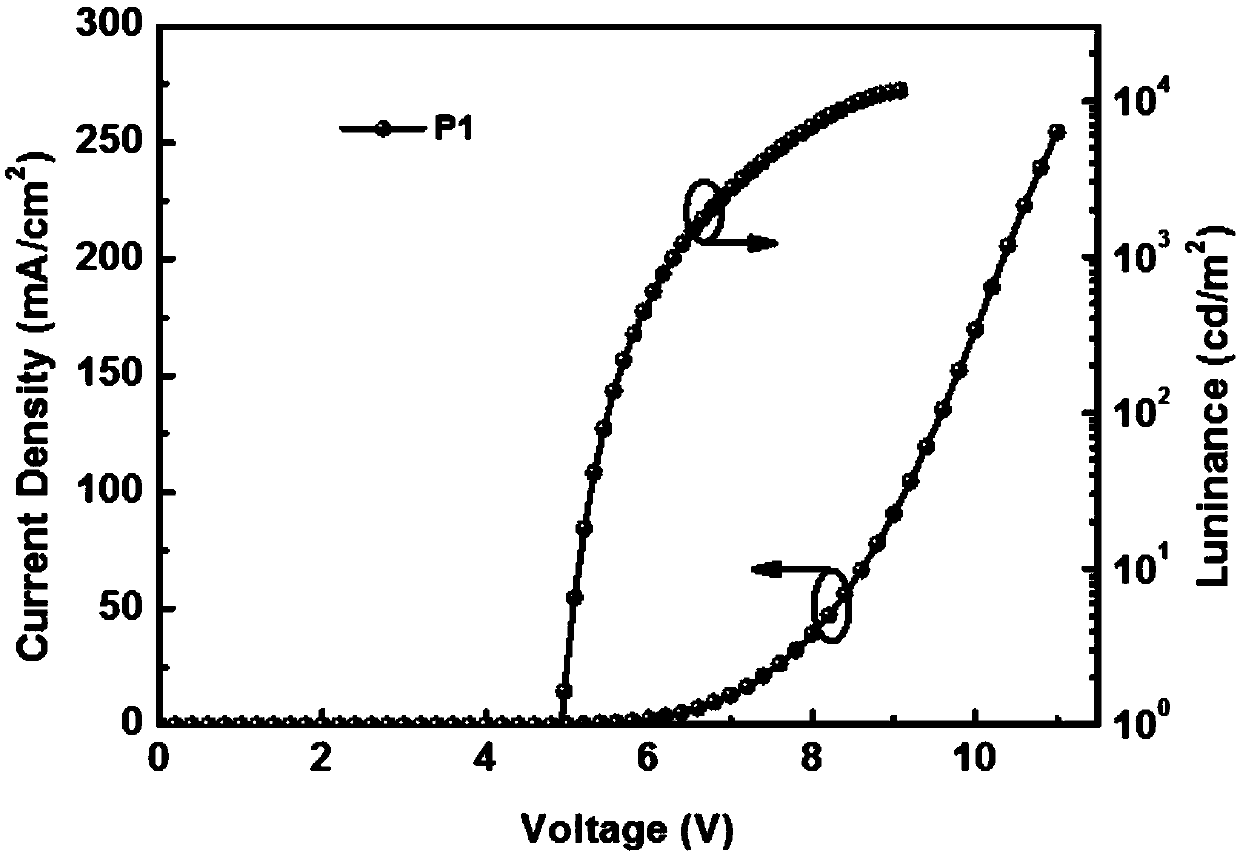
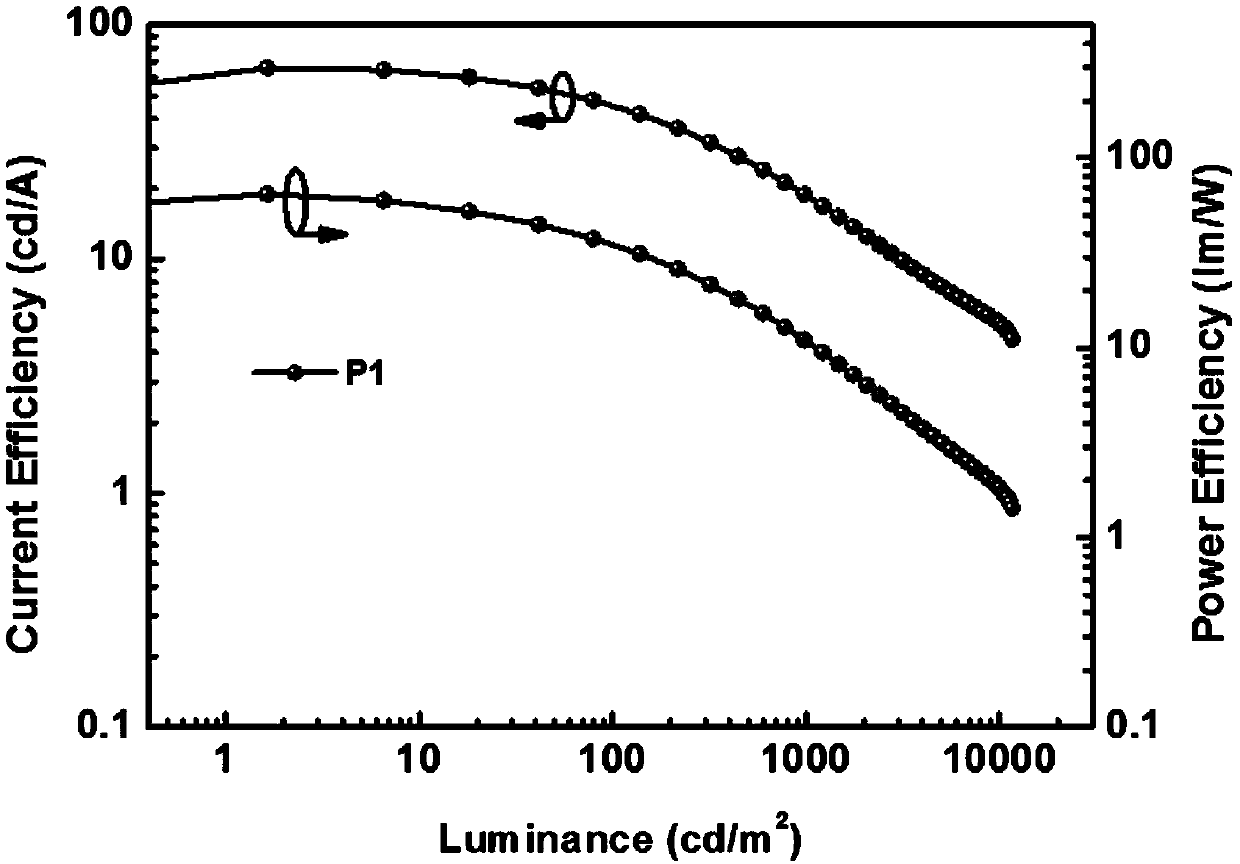
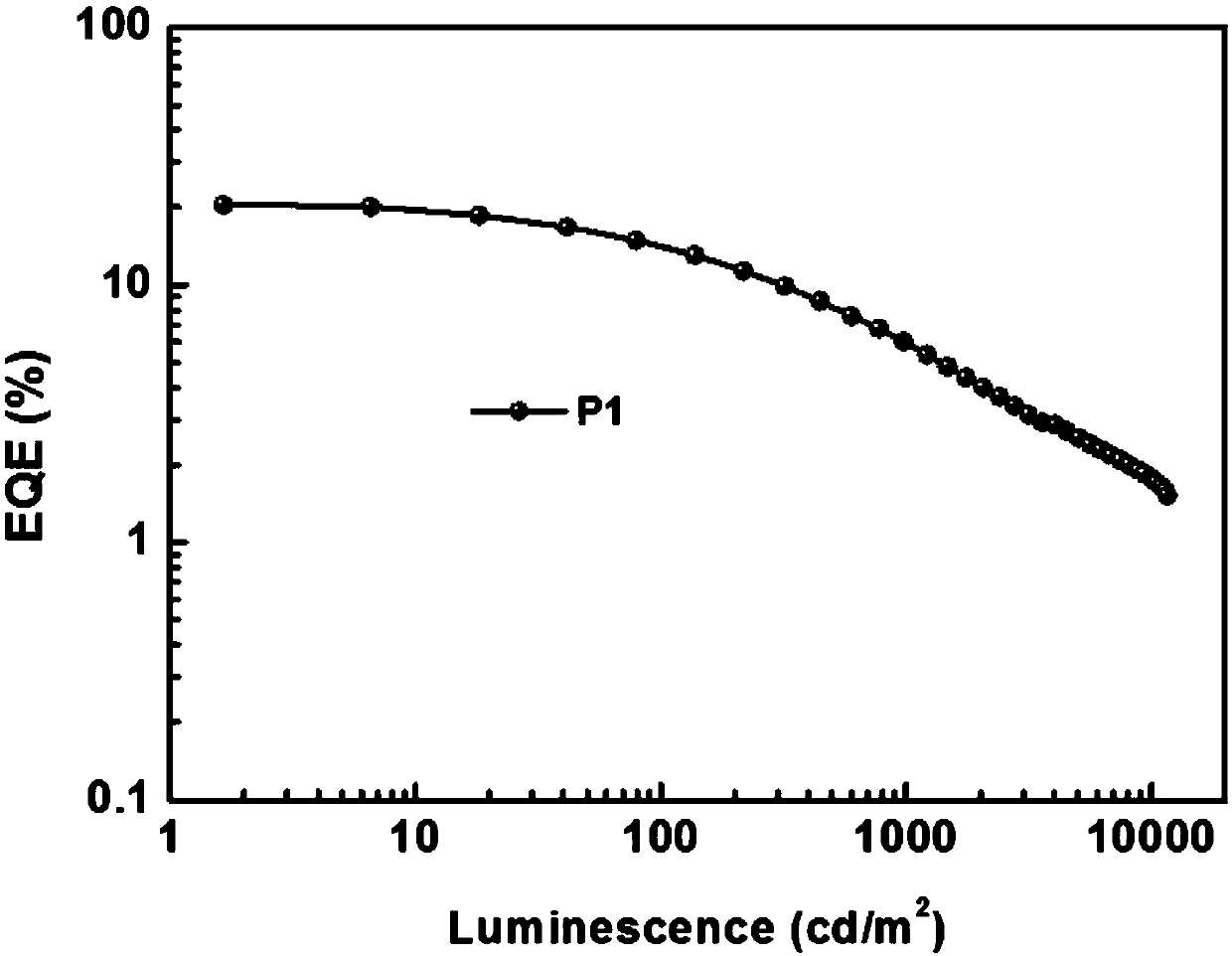
Small-molecule luminescent materials based on 1,3-benzodiazine (quinazoline) and production method and application of small-molecule luminescent materials based on 1,3-benzodiazine (quinazoline)
A technology of luminescent materials and phthalazine, which is applied in the direction of luminescent materials, chemical instruments and methods, semiconductor/solid-state device manufacturing, etc., and can solve problems such as low efficiency
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0051] Synthesis of small molecule luminescent material P1 based on 1,3-naphthyridine (quinazoline):
[0052]
[0053] (1) Dissolve phenoxazine (1.66g, 5.00mmol), sodium tert-butoxide (0.96g, 10.00mmol) in 80mL1,4-dioxane, stir at room temperature for 30min under a nitrogen atmosphere, then add CuI ( 0.10g, 0.50mmol), 1,10-phenanthroline (0.10g, 0.50mmol), p-bromoiodobenzene (1.69g, 6.00mmol), react at 110°C for 24h. Obtain white solid product and obtain product 1, productive rate 75%, reaction formula is as follows:
[0054]
[0055] (2) Dissolve the product 1 (5.0g, 10.2mmol) in 400mL of anhydrous tetrahydrofuran, and at -78°C, add 4.7mL of n-butyllithium (2.5mol / L, 12.30mmol) dropwise, at -78°C Under reaction 1h. Then 2.30g 2-isopropoxy-4,4,5,5-tetramethyl-1,3,2-dioxaborolane (2.3g, 12.3mmol) was added dropwise and reacted overnight at room temperature , to obtain white solid product 2 with a yield of 73%, the reaction formula is as follows:
[0056]
[0057] (...
Embodiment 2
[0060] Synthesize small molecule luminescent material P2 based on 1,3-naphthyridine (quinazoline), the reaction formula is as follows:
[0061]
[0062] With embodiment 1, replace 4-phenyl-2-chloro-quinazoline with 2-chloro-quinazoline (0.20g, 1.5mmol), obtain based on 1,3-naphthyridine (quinazoline) The yield of small molecule luminescent material P2 is about 81%.
Embodiment 3
[0064] Synthesis of small molecule luminescent material P3 based on 1,3-naphthyridine (quinazoline), the reaction formula is as follows:
[0065]
[0066] With embodiment 1, replace 4-phenyl-2-chloro-quinazoline with 4-chloro-quinazoline (0.20g, 1.5mmol), obtain based on 1,3-naphthyridine (quinazoline) The small molecule luminescent material P3 has a yield of about 89%.
PUM
| Property | Measurement | Unit |
|---|---|---|
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com