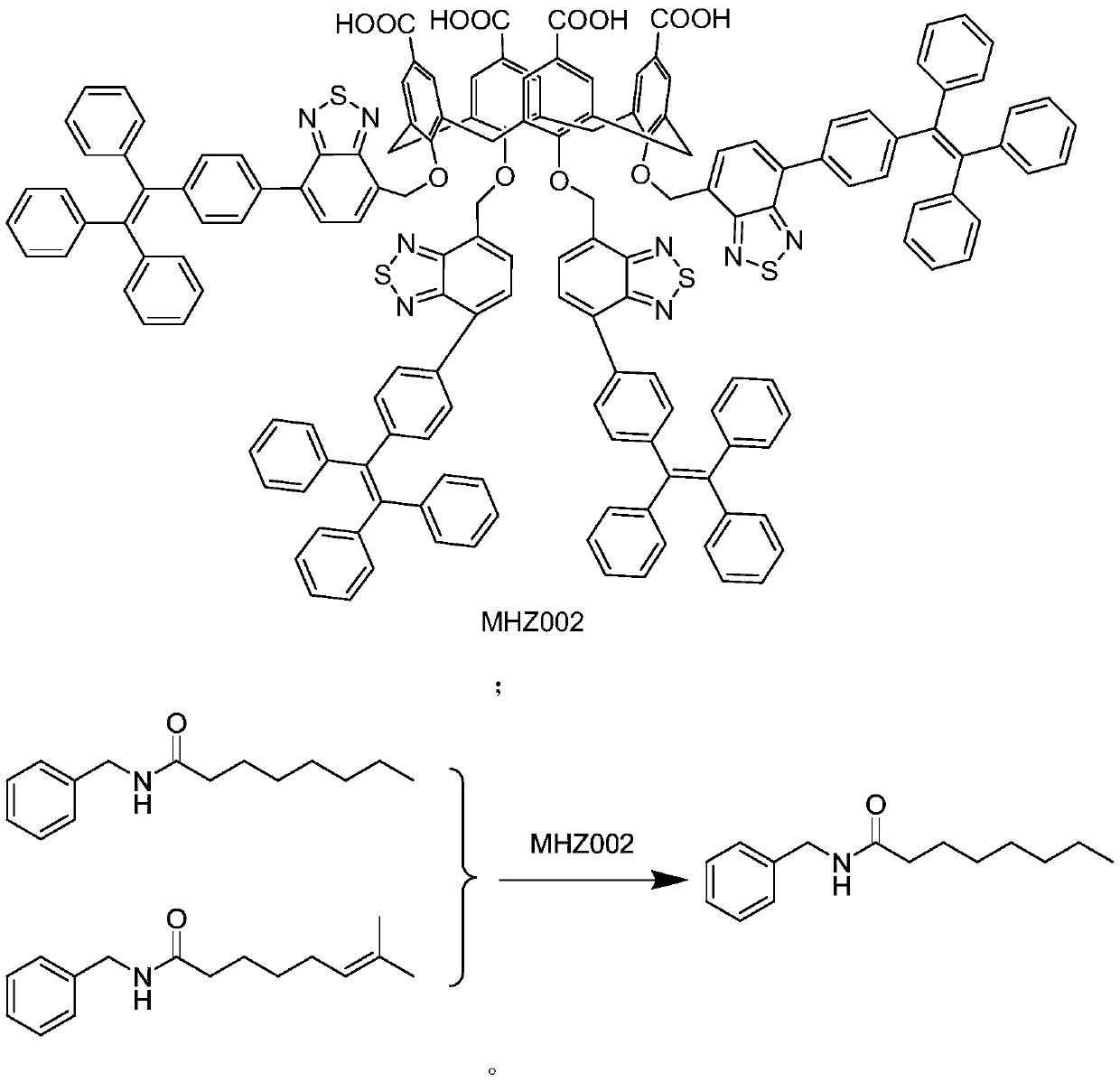
Refining method for synthesizing high-purity capsaicine
A technology for synthesizing capsaicin and a refining method, which is applied in directions such as separation/purification of carboxylic acid amides, organic chemistry, etc., can solve the problems of unsuitability for industrial production, low efficiency, large energy consumption, etc., and achieves good cycle repeatability and atom economy. high effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
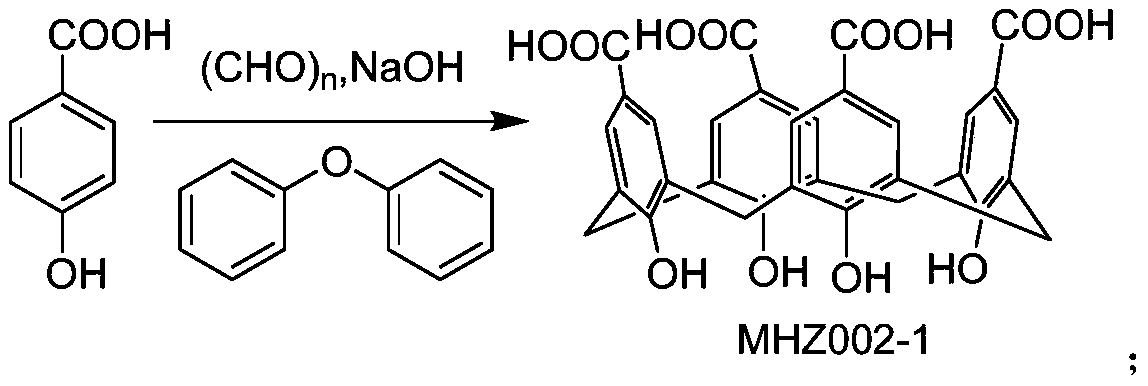
[0025] Preparation of MHZ001-1
[0026] Add 23ml 37% formaldehyde solution, 36.8g p-hydroxybenzoic acid and 4.6g caustic soda in 250ml there-necked bottle. Under the condition of magnetic stirring, use a heating mantle to raise the temperature to 110°C, evaporate the water until the system in the bottle becomes a purple jelly, and let it stand to cool to room temperature. Add 40ml of diphenyl ether, raise the temperature to 170°C, bubble the solution with argon until the solution turns dark green, reflux for two hours, and let it stand to cool to room temperature. Add 50ml of ethyl acetate, stir for 30 minutes to crystallize. Suction filtration, beating and rinsing the filter cake with water to obtain MHZ001-1 as 2.00 g of white solid.
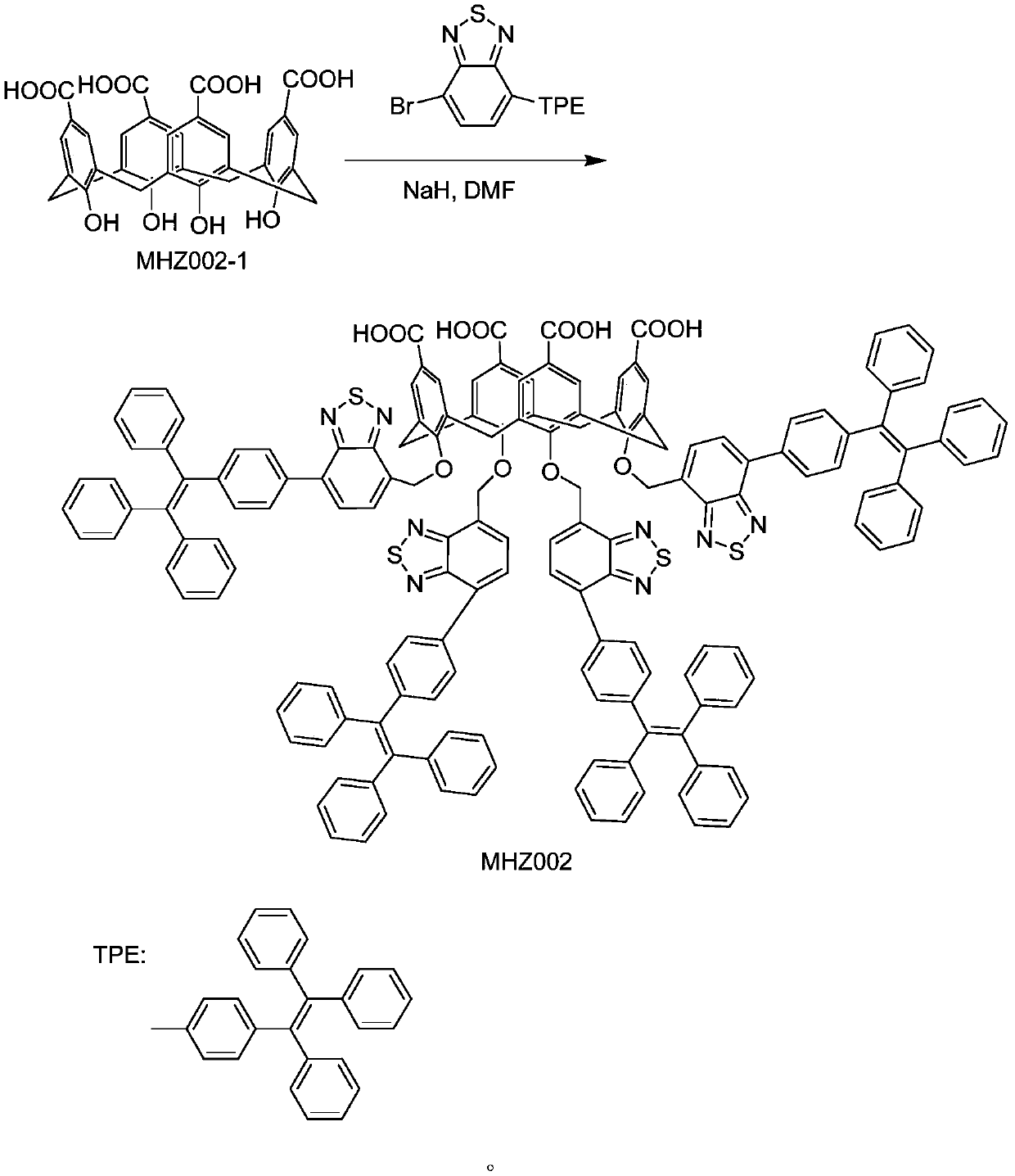
[0027] Preparation of MHZ001
[0028] Add 0.5 g of MHZ001-1, 10.64 g of 4-bromo-7-tetraphenylethylene benzothiadiazole, and 20 ml of DMF into a 50 ml three-necked flask, stir at room temperature for 15 minutes, add 1.23 g of sodium hydride ...
Embodiment 1
[0030] Take 24.5 g (0.1 mol) of (trans) 8-methyl-N-vanillyl-6-nonenamide and add 24.5 g (0.1 mol) of synthetic capsaicin with a total weight of 2450 g into a 10 L three-necked bottle, and add 251.2 g (0.1 mol) ( MHZ001) calixarene, add 2450g of dichloromethane, stir and react at room temperature for 6 hours. After the solution changed from green to red, dichloromethane was distilled off at 30 °C and -0.01 MPa, 2450 g of diethyl ether was added, stirred at -5 °C for a period of time, filtered, and the filter cake was dried to obtain high-purity capsaicin with an HPLC purity of 99.8%. The filtrate is to be processed to recover MHZ001.
Embodiment 2
[0032] Take 24.5g (0.1mol) of (trans) 8-methyl-N-vanillyl-6-nonenamide and add 24.5g (0.1mol) of capsaicin with a total weight of 2450g into a 10L three-necked bottle, and add 251.2g (0.1mol) of MHZ001, 7350 g of dichloromethane was added, and the reaction was stirred at room temperature for 8 hours. After the solution changed from green to red, dichloromethane was distilled off at 30°C and -0.01MPa. Added 7350g of diethyl ether, stirred at 5°C for a period of time, filtered, and the filter cake was dried to obtain high-purity capsaicin with an HPLC purity of 99.8%. The filtrate is to be processed to recover MHZ002.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com