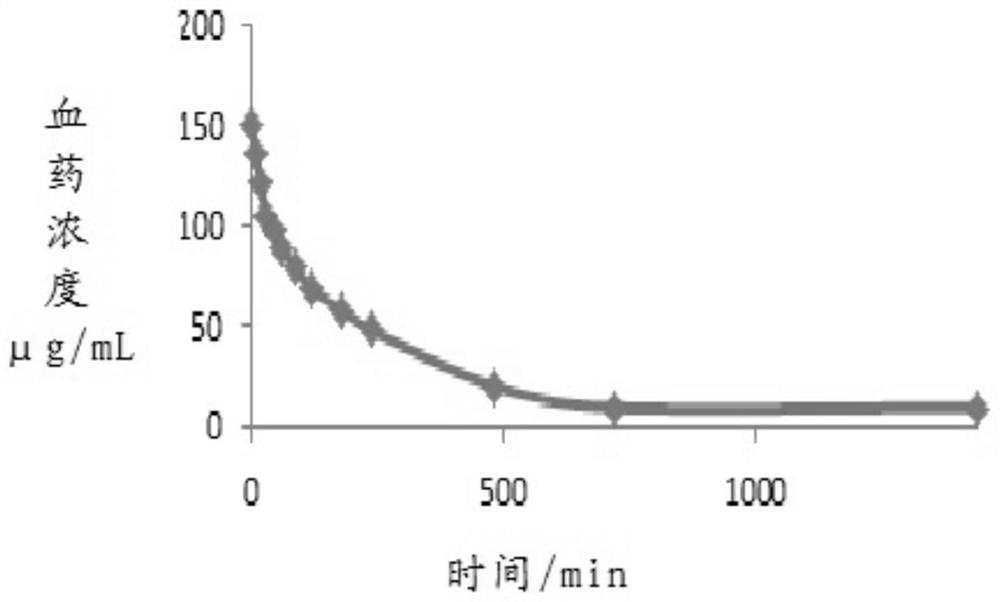
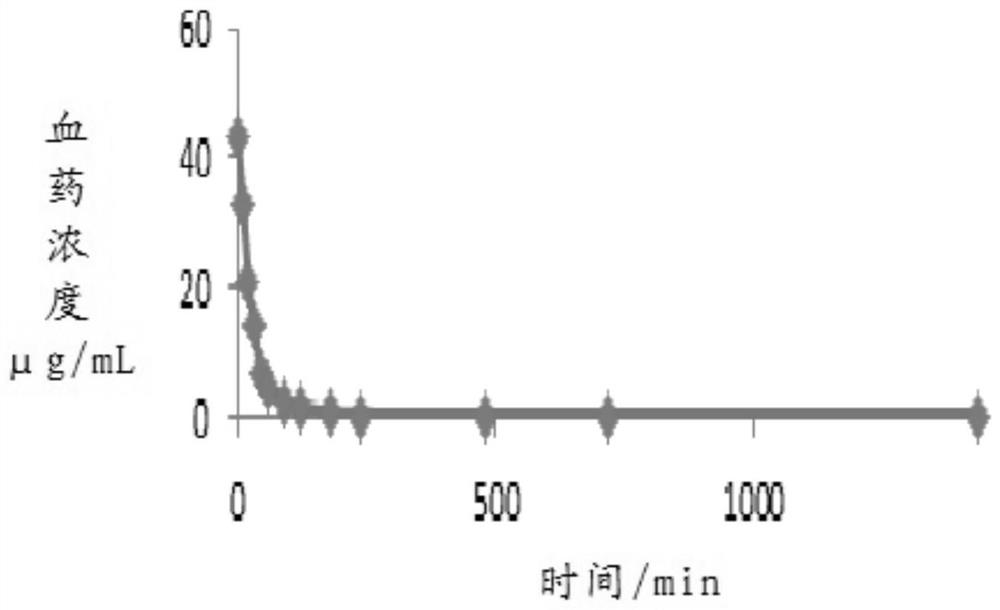
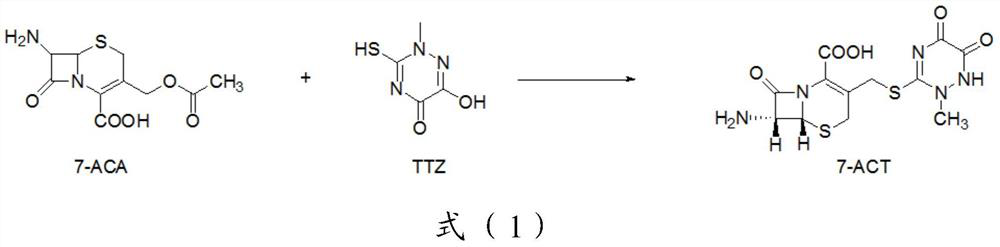
Trisofen Ceftriaxone Sodium Compound Drug Preparation and Its New Indication for Treatment of Pelvic Inflammation
A technology of ceftriaxone sodium and solvent, which is applied in the field of drug preparation, can solve the problems of potential safety hazards, no therapeutic effect, toxicity, etc., and achieves the effects of limited reaction temperature reduction, improved clinical treatment effect, and accelerated reaction progress.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0126] The synthesis of embodiment 1 ceftriaxone sodium
[0127] Preparation of 7-ACT:
[0128] Step 1: In a reaction flask, disperse 544g (2mol) 7-ACA in 2000ml of dichloromethane, add 334g (2.10mol) of TTZ, 284g (2.2mol) of N,N-diisopropylethylamine, stir and cool down To below 5°C, add TiCl dropwise to the reaction system 4 378g (2mol), tetrabutyl titanate 102g (0.30mol), after the addition is complete, keep warm for 4h;
[0129] After the reaction, add 10% sodium bicarbonate solution dropwise to the reaction system to adjust the pH to 7.5, separate the liquids, extract the water phase once with dichloromethane, add glacial acetic acid dropwise to the water phase, adjust the pH to 2.0, and cool to Below 5°C, crystals were precipitated, suction filtered, and dried to obtain 704g of 7-ACT, with a yield of 95%;
[0130] Preparation of ceftriaxone sodium crude product:
[0131] Step 2: Add 371g (1.0mol) of 7-ACT prepared in step 1, 368g (1.05mol) of AE-active ester, and 15...
Embodiment 2
[0135] The synthesis of embodiment 2 ceftriaxone sodium
[0136] The same as the synthesis process of Example 1, the only difference is: in step 1), TiCl is added dropwise to the reaction system 4 340g (1.8mol), tetrabutyl titanate 61g (0.18mol), ie TiCl 4 The molar ratio with 7-ACA is 0.9:1, TiCl in the catalyst 4 The molar ratio to tetrabutyl titanate is 1:0.1.
Embodiment 3
[0137] The synthesis of embodiment 3 ceftriaxone sodium
[0138] The same as the synthesis process of Example 1, the only difference is: in step 1), TiCl is added dropwise to the reaction system 4 416g (2.2mol), tetrabutyl titanate 150g (0.44mol), namely TiCl The mol ratio of and 7-ACA is 1.1:1, TiCl in the catalyst 4 The molar ratio to tetrabutyl titanate is 1:0.2.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com