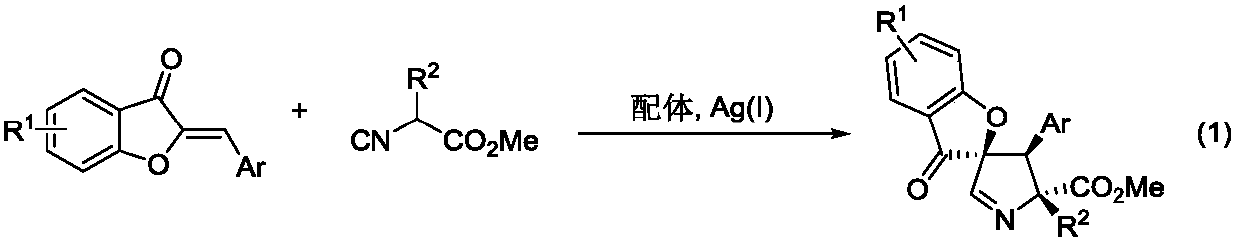
Asymmetric synthesis method for pyrroline derivative with spirane structure
A synthetic method and technology of spiro ring structure, applied in the field of asymmetric synthesis of pyrroline derivatives, achieving high stereoselectivity, simple operation and high yield
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0030] Preparation of compound 3a
[0031]
[0032] step:
[0033]
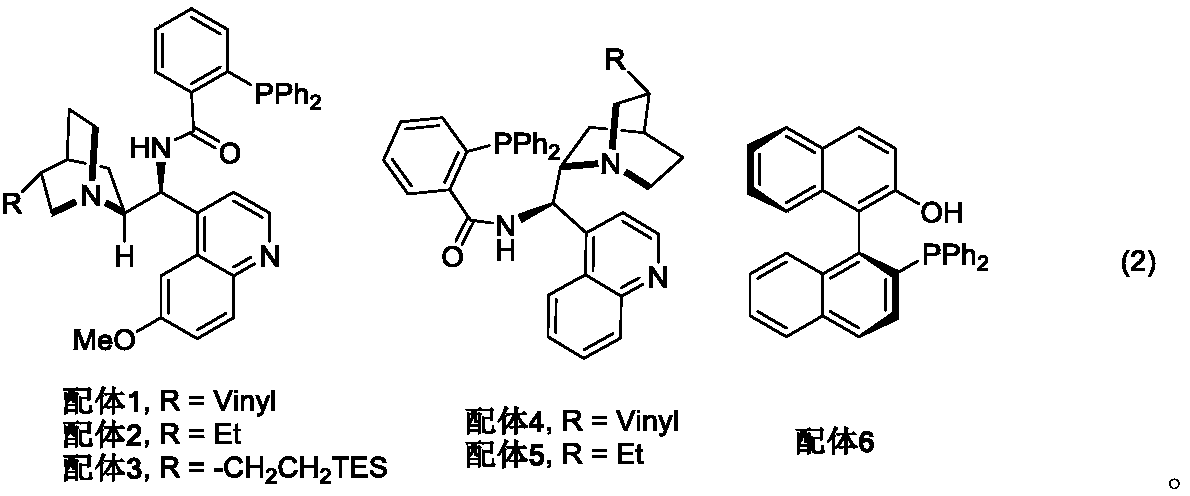
[0034] Accurately weigh Ligand 1 (6.1 mg, 0.01 mmol) and silver acetate (0.83 mg, 0.005 mmol) into a 10 mL reaction tube equipped with a stirrer, add 2 mL of dichloromethane and stir at 25°C for 5 minutes. Then isocyanoacetate 2a (22.68mg, 0.12mmol) and starting material 1a (22.2mg, 0.1mmol) were added and reacted at 25°C, monitored by TLC until the reaction of starting material 1a was complete. The reaction solution was directly concentrated, and separated and purified by column chromatography to obtain 34.9 mg of product 3a with a yield of 85%.
[0035] White solid, melting point 58-59℃, R f =0.2 (petroleum ether: ethyl acetate = 3:2). 1 H NMR (400MHz, Chloroform-d) δ7.68(s,1H),7.65–7.60(m,2H),7.38(dd,J=6.5,3.1Hz,2H),7.29–7.16(m,9H), 7.09(t, J=7.5Hz, 1H), 4.01(s, 1H), 3.62(s, 3H), 3.08(d, J=13.4Hz, 1H), 2.97(d, J=13.4Hz, 1H); 13 C NMR(101MHz,Chloroform-d)δ197.14,172.04,171.39,160.38,138.79,135.82...
Embodiment 2
[0038] Preparation of compound 3b
[0039]
[0040] step:
[0041]
[0042]Ligand 2 (12.3mg, 0.02mmol) and silver oxide (2.3mg, 0.01mmol) were accurately weighed and placed in a 10mL reaction test tube equipped with a stirring bar, 1mL of tetrahydrofuran was added and stirred at 0°C for 5 minutes. Then isocyanoacetate 2a (22.68mg, 0.12mmol) and starting material 1b (25.2mg, 0.1mmol) were added and reacted at 0°C, monitored by TLC until the reaction of starting material 1b was complete. The reaction solution was directly concentrated, and separated and purified by column chromatography to obtain 43.6 mg of product 3b with a yield of 99%.
[0043] White solid, melting point 81-82°C, R f =0.3 (petroleum ether:ethyl acetate=5:1). 1 H NMR (400MHz, Chloroform-d) δ7.68 (s, 1H), 7.49 (d, J = 8.6Hz, 1H), 7.39–7.37 (m, 2H), 7.23 (ddd, J = 20.8, 13.8, 6.4 Hz,8H),6.62(dt,J=7.3,3.6Hz,1H),6.58(d,J=1.5Hz,1H),4.00(s,1H),3.87(s,3H),3.61(s,3H ), 3.07(d, J=13.4Hz, 1H), 2.95(d, J=13.4H...
Embodiment 3
[0046] Preparation of compound 3c
[0047]
[0048] step:
[0049]
[0050] Accurately weigh ligand 3 (21.8 mg, 0.03 mmol) and silver carbonate (4.1 mg, 0.015 mmol) into a 10 mL reaction tube equipped with a stirrer, add 0.5 mL of chloroform and stir at -20°C for 5 minutes. Then isocyanoacetate 2a (22.68mg, 0.12mmol) and starting material 1c (30.1mg, 0.1mmol) were added and reacted at -20°C, monitored by TLC until the reaction of starting material 1c was complete. The reaction solution was directly concentrated, and separated and purified by column chromatography to obtain 44.1 mg of product 3c with a yield of 90%.
[0051] White solid, melting point M.P.73-75℃, R f =0.5 (petroleum ether:ethyl acetate=5:1). 1 H NMR (400MHz, Chloroform-d) δ7.67(s,1H),7.45(d,J=8.2Hz,1H),7.38(s,1H),7.34–7.33(m,2H),7.30–7.28( m,3H),7.24–7.21(m,4H),7.19–7.15(m,2H),3.99(s,1H),3.60(s,3H),3.06(d,J=13.4Hz,1H),2.93 (d,J=13.4Hz,1H); 13 C NMR(101MHz,Chloroform-d)δ195.87,171.94,171.34,159.83,13...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com