Application of vinblastine III in preparing medicine for preventing or treating Alzheimer's disease
A technology for Alzheimer's disease and vinblastine, which is applied in the field of new drug development and can solve problems such as poor curative effect, toxic and side effects, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0032] Example 1 Cell Experiment
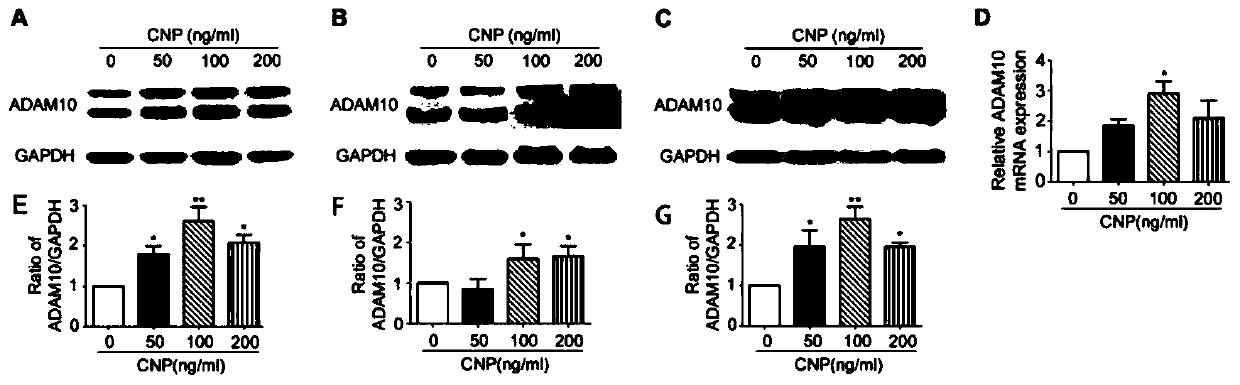
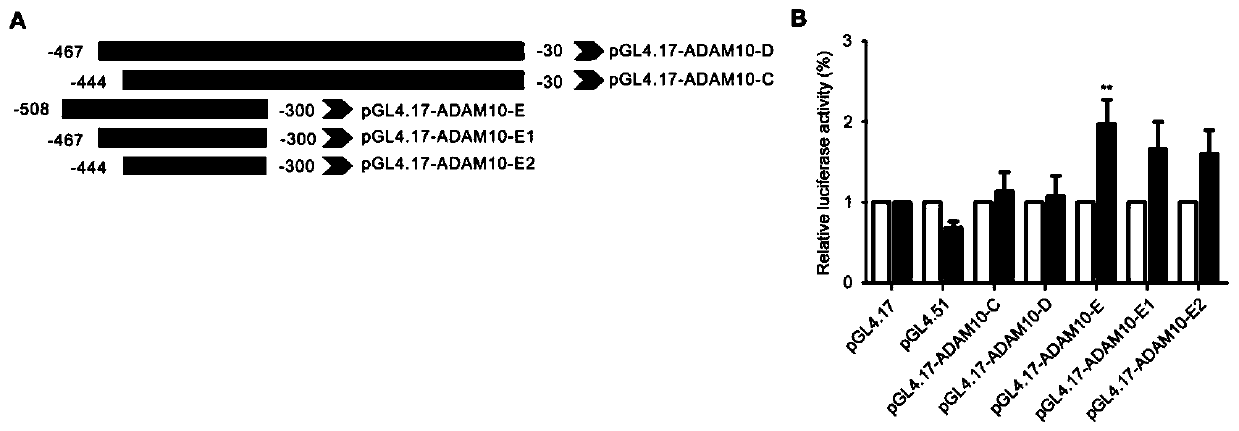
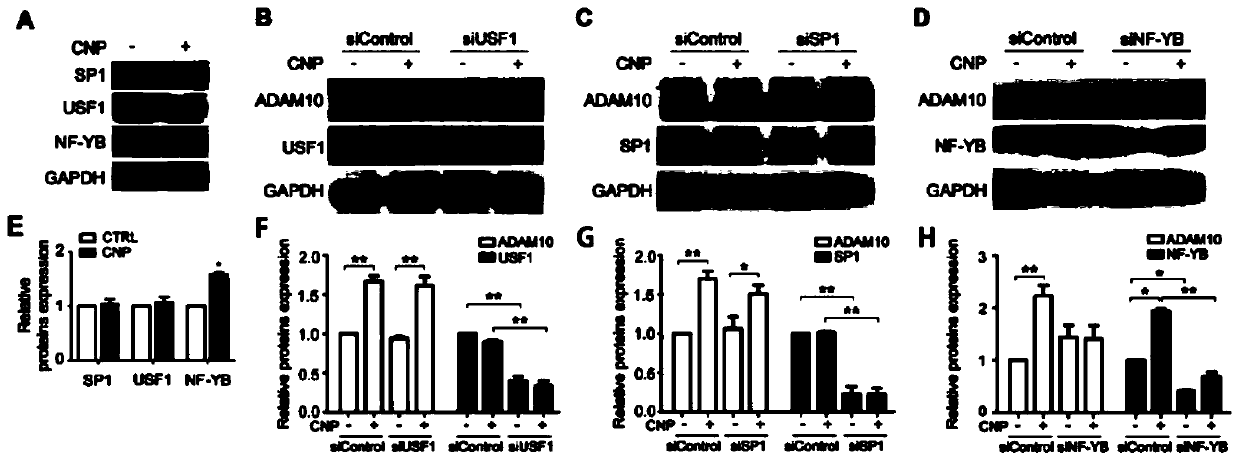
[0033] CNP increases the expression of ADAM10 through the NF-YB pathway
[0034] Human embryonic kidney cells HEK293, human neuroblastoma cells SH-SY5Y, and mouse hippocampal neuron cells HT22 were seeded in a 6-well plate. HEK293 cells were 2×10 per well 5 Y5Y cells 4×10 per well 5 HT22 cells 2×10 per well 5 A. The cell fusion rate of HEK293 and HT22 cells is about 60%-70%, and the cell fusion rate of Y5Y cells is about 80%-90%. After treatment for 24 hours, the concentration of the drug is 0ng / ml and 50ng / ml respectively. ,100ng / ml,200ng / mL. The experiment verified that the concentration of 100ng / mL was safe, stable and effective, and the subsequent experiments all chose 100ng / mL as the drug treatment concentration.
[0035] In order to verify the expression of transcription and protein levels, RT-PCR and Western blot (WB) experiments were performed. Specific experimental operations:
[0036] RT-PCR
[0037] RNA extraction: 1mL RNAiso Plus / well (6-...
Embodiment 2
[0071] Example 2 Animal experiment
[0072] Experimental animals: APP / PS1 transgenic mice and littermate wild-type mice used in the experiment were all derived from breeding, and their parents were purchased from the JACKSON laboratory in the United States. The mice were raised in the SPF transgenic mouse surrogate room of Chongqing Medical University. All animal feeding procedures were in accordance with the "Chinese Laboratory Animal Management Regulations." All animal experiments strictly abide by the "Animal Use Policy" promulgated by the Chinese Neuroscience Association in 1995.
[0073] experimental method
[0074] The experiment is divided into four groups: WT+CTRL, WT+CNP, AD+CNP, AD+CNP.
[0075] Conophylline (CNP for short) drug treatment method: The experimental group of 6-month-old AD mice and WT of the same age were injected intraperitoneally at the same time every other day with 1μg / KgCNP. The control group mice were given the same amount of DMSO (Dimethyl sulfoxide, di...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com