Composition containing azabicyclic derivatives and application thereof
A technology of azabicyclo and composition, which is applied in the direction of medical preparations containing active ingredients, drug combinations, plant raw materials, etc., and can solve problems such as strong adverse reactions and drug resistance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
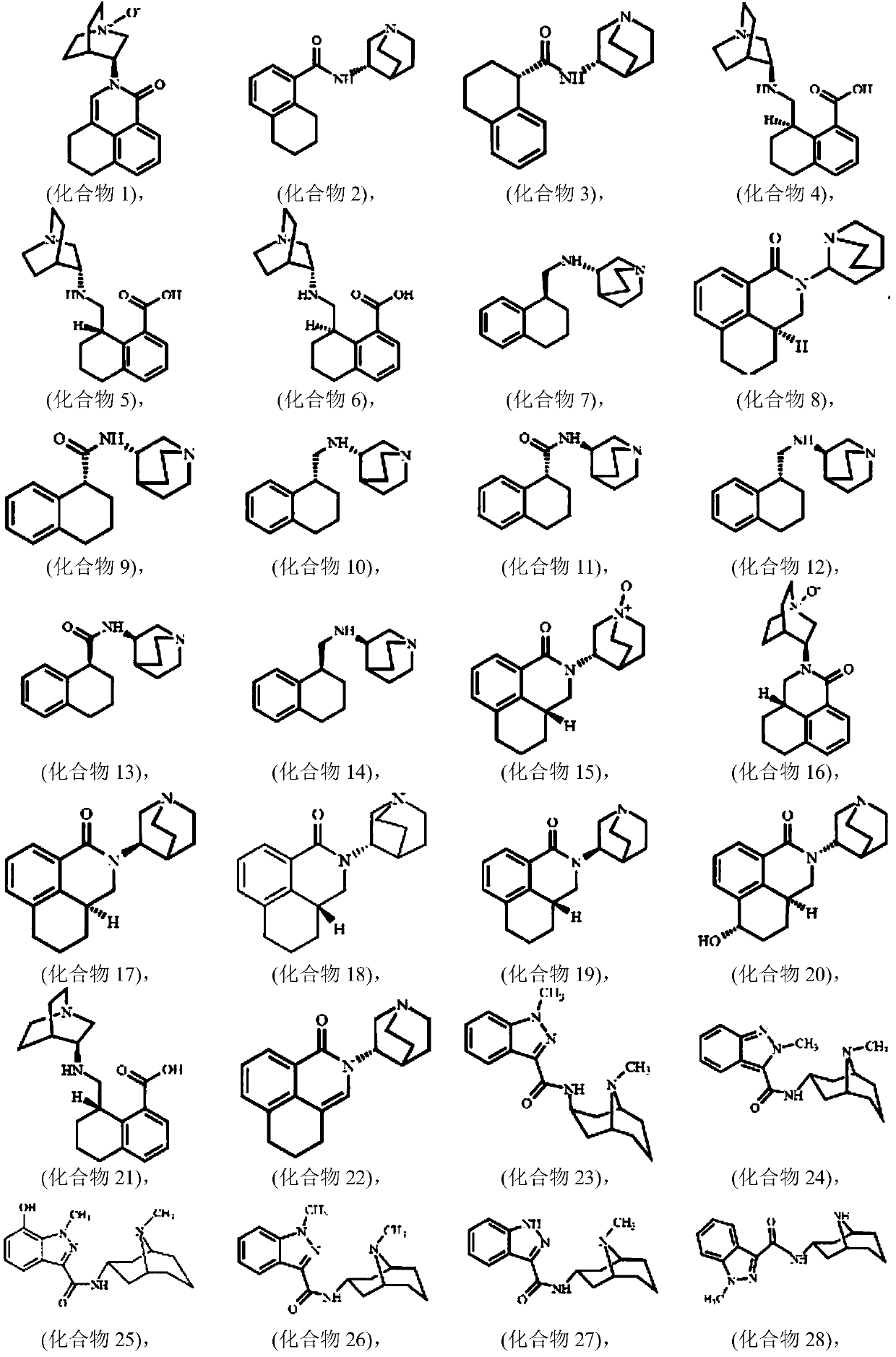
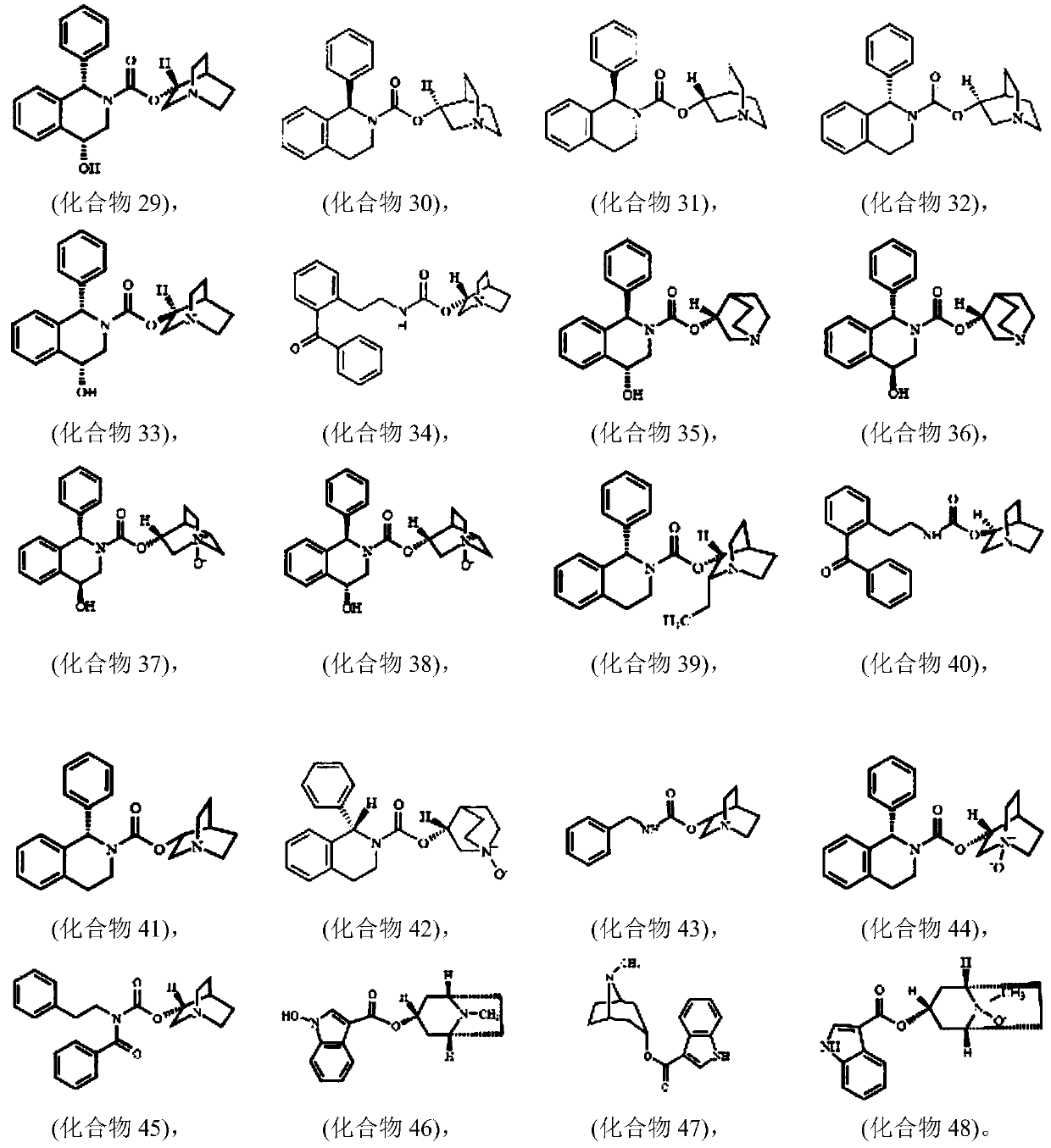
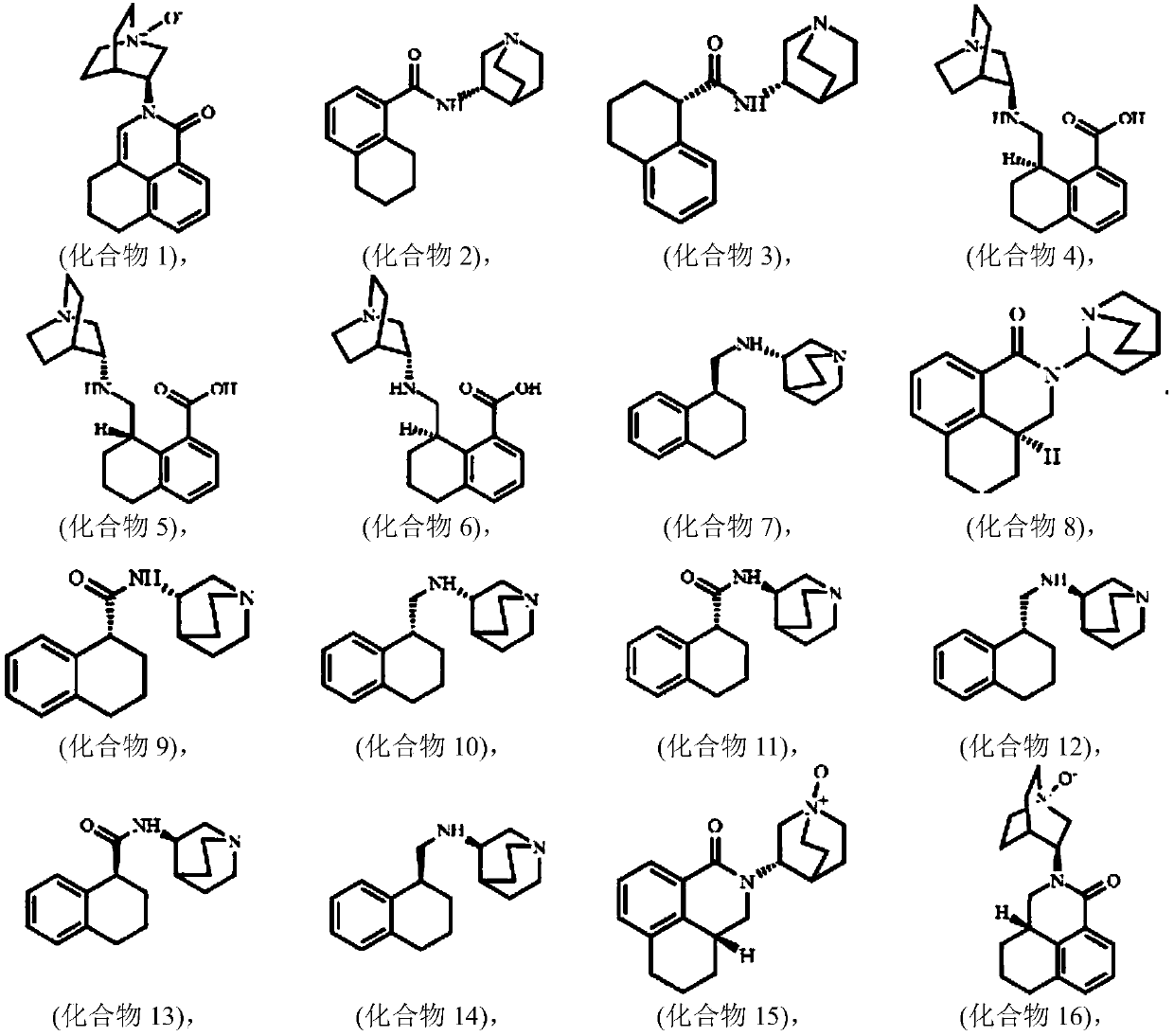
Image
Examples
preparation example 1
[0020] The preparation of preparation example 1 plant extract
[0021] Ultrasonic extraction method is used to dry the shade-dried plant material at 40°C, crush it, pass through a 40-mesh sieve, and put it into a ziplock bag. Before the test, 50 g of plant dry powder was weighed, and 10 to 20 times (v / w) of water was added for ultrasonic extraction, 60 min each time, and the extraction was repeated 3 times. Concentrate after filtration and store in a refrigerator at 4°C until use.
[0022] The plant material is selected from the group consisting of blueberry (PE1), stone see wear (PE2), grifola frondosa (PE3), eye tree lotus (PE4), sessile five plus (PE5), goldenflower sunflower (PE6), golden camellia (PE7), Snakevine (PE8), Pinellia (PE9), Hedyotis diffusa (PE10), Zhemu (PE11), Ashen (PE12), African Malus (PE13), Jade Fungus (PE14), It is one of Euphorbia (PE15), Brown Algae (PE16), Clover (PE17) and Tibetan Capillary (PE18).
Embodiment 1
[0098] Example 1 Preparation of Liquid Preparations Containing Azabicyclic Derivatives and Polyhydroxyl Compounds
[0099] Prescription (10000 unit dose)
[0100]
[0101]
[0102] Note:
[0103] a Represents no added plant extracts
[0104] Preparation Process
[0105] Add the prescribed amount of citric acid, mixture X-Y-PEZ, edetate disodium, sodium chloride, benzalkonium chloride, and mannitol, add 20% of the prescribed amount of water for injection to dissolve, and filter through a 0.22 μm microporous membrane, Use sodium hydroxide to adjust the pH value to about 6.3, pass through a 220-mesh sieve, seal and boil for 60 minutes, cool to room temperature, and add water for injection to the full amount.
Embodiment 2
[0106] Embodiment 2 Preparation of oral solid preparation containing a mixture of azabicyclic derivatives
[0107] Prescription (1000 unit dose)
[0108]
[0109]
[0110] Preparation
[0111] Take the mixture X-Y-PEZ and auxiliary materials in the prescribed amount, and pass through a 100-mesh sieve. Take the mixture X-Y-PEZ, lactose, microcrystalline cellulose, crospovidone and starch and mix thoroughly; take the prescribed amount of hypromellose, and prepare a solution with a concentration of 10% based on hypromellose, Use lactic acid to adjust the pH to 3.0-4.0, add it to the above-mentioned mixture to make a soft material, granulate with a 16-mesh sieve, and dry at 80°C for 3-4 hours. Use a 16-mesh sieve to sieve the granules, add the prescribed amount of micropowder silica gel and magnesium stearate, mix well, fill the capsules, and obtain capsules;
[0112] Take the mixture X-Y-PEZ and auxiliary materials in the prescribed amount, and pass through a 100-mes...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com