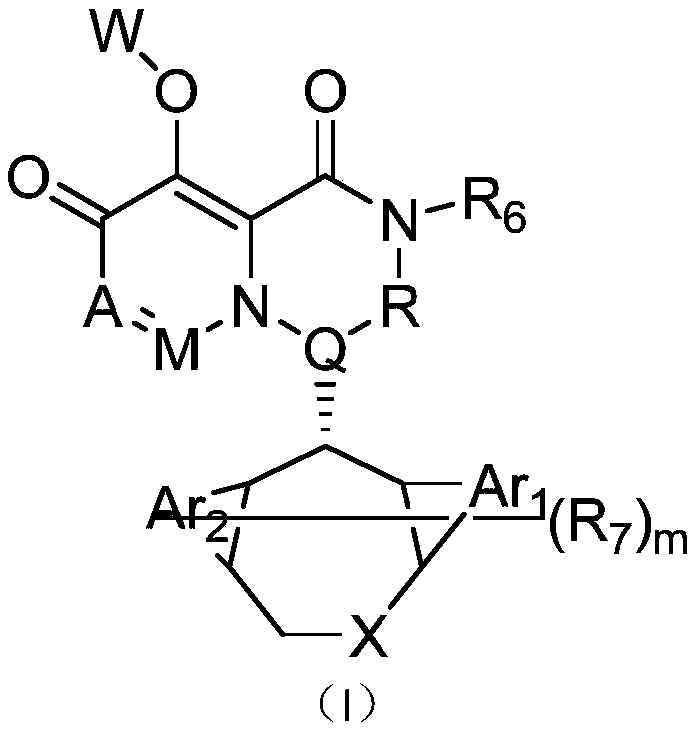
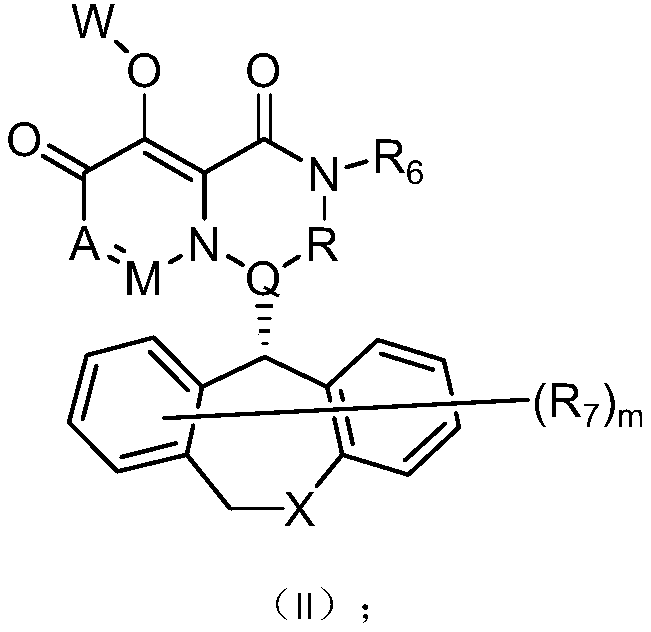
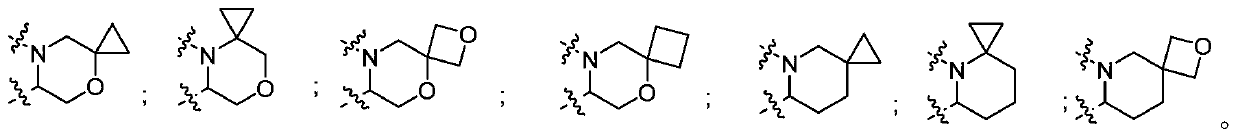
Pyridone derivative, composition thereof and application thereof as Anti-influenza drug
一种衍生物、吡啶酮的技术,应用在吡啶酮衍生物、其组合物及作为抗流感病毒药物的应用领域,能够解决重症患者疗效可疑等问题
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0192] Embodiment 1: preparation compound I-1
[0193]
[0194] Preparation of Compound 1b: Compound 1a (2.0 g, 8.1 mmol), DBU (1.85 g, 12.2 mmol) and ethyl iodide (2.28 g, 14.6 mmol) were reacted in 20 mL of DMF at room temperature for 16 hours. Then add 100mL water to dilute and extract with EA. The organic phases were combined, washed successively with sodium thiosulfate, 0.5N HCl and saturated brine, dried over anhydrous sodium sulfate and spin-dried to obtain 2.1 g of an oily product, namely compound 1b.
[0195]The preparation of compound 1c: compound 1b (2.1g, 7.7mmol), Boc hydrazine (1.53g, 11.6mmol) and pyridine p-toluenesulfonate (5.78g, 23.1mmol) in N,N-dimethylacetamide (20mL ) at 60°C for 16 hours. After the reaction was completed, 100 mL of water was added to the reaction liquid, and then extracted with ethyl acetate (50 mL×3). The organic phases were combined, washed with saturated brine, dried over anhydrous sodium sulfate, and concentrated. The crude pro...
Embodiment 2
[0202] Embodiment 2: preparation compound 1-5
[0203]
[0204] Preparation of compound 2b: compound 1h (180mg, 0.49mmol) and 2a (264mg, 1.0mmol) were reacted in a solution of T3P in ethyl acetate at 100°C for 3 hours in a sealed manner. Cool, add saturated NaHCO 3 The aqueous solution was diluted and extracted with ethyl acetate. The organic phases were combined, dried, concentrated, and separated on a preparative plate to obtain 190 mg of product. ESI-MS m / z 612.2(M+H) + .
[0205] Preparation of compound I-5: compound 2b (190 mg, 0.31 mmol) and lithium chloride (50 mg, 1.18 mmol) were reacted in 5 mL DMA at 100° C. for 3 hours. After the reaction is complete, add 10 mL of water to dilute, and adjust the pH to 5-6 with 2N hydrochloric acid. After filtration, the solid was sucked dry to obtain 136 mg of product. 1 HNMR (400MHz, CDCl 3 )δ: 7.04-7.12(m, 3H), 7.00-7.02(d, 1H, J=7.6Hz), 6.90-6.93(m, 1H), 6.79-6.83(m, 1H), 6.63-6.64(d, 1H, J=7.2Hz), 5.74-5.76(d, 1H, J=7...
Embodiment 3
[0206] Embodiment 3: preparation compound I-7
[0207]
[0208] Preparation of compound 3b: Compound 3a (5.0g, 27.8mmol) was added into n-butyl vinyl ether (10mL), then palladium trifluoroacetate (100mg, 0.3mmol), triethylamine (3.03g, 30mmol) and DPPP (124mg, 0.3mmol), closed the reaction, stirred at 75°C overnight, TLC showed that the reaction was complete. Add 50mL of water, extract twice with ethyl acetate, wash the organic phase with saturated brine and dry over anhydrous sodium sulfate, concentrate and separate by column chromatography to obtain 4.8g of the product, which is directly used in the next step.
[0209] Preparation of compound 3c: Compound 3b (4.8g, 23.3mmol) was dissolved in 50mL of anhydrous toluene, and 1N diethylzinc solution (70mL, 70mmol) was added at -40°C under nitrogen protection. After the addition was completed and the reaction was stirred for 1 h, chloroiodomethane (8.22 g, 46.6 mmol) was added. After the addition, continue to stir the reacti...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com