Non-noble metal hydrazine oxidation catalyst based on collaborative modification and preparation method thereof
A technology for oxidation catalysts and non-precious metals, applied in nanotechnology for materials and surface science, electrical components, battery electrodes, etc., can solve the problems that the catalytic performance of catalysts cannot meet the requirements of practical applications, and achieve high intrinsic catalytic activity and good Conductivity, effect of improving mass transfer performance
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
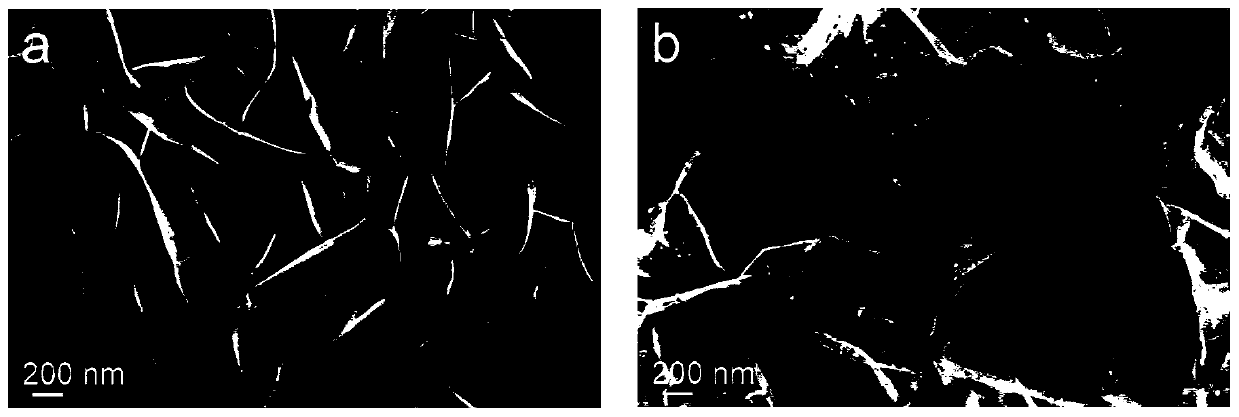
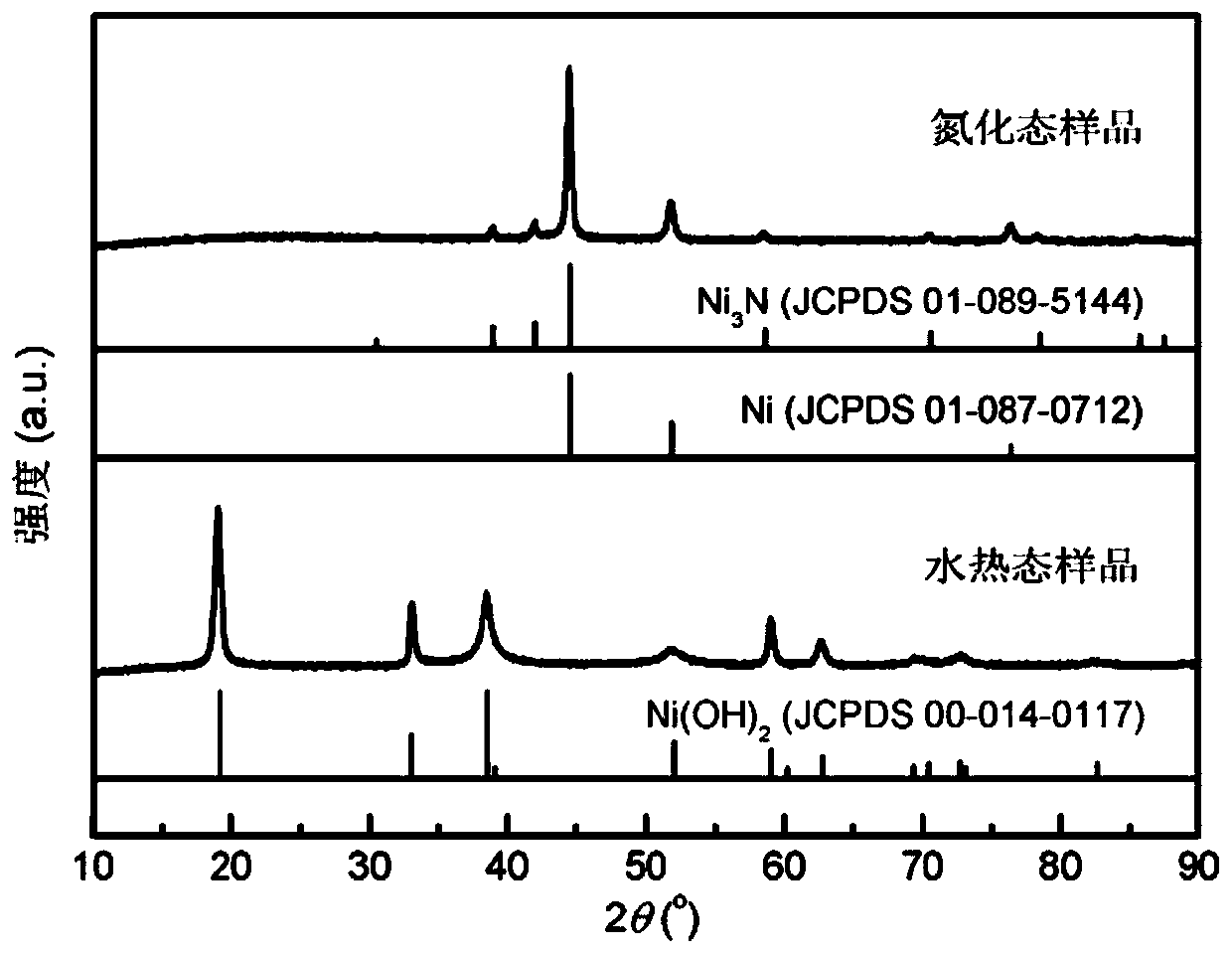
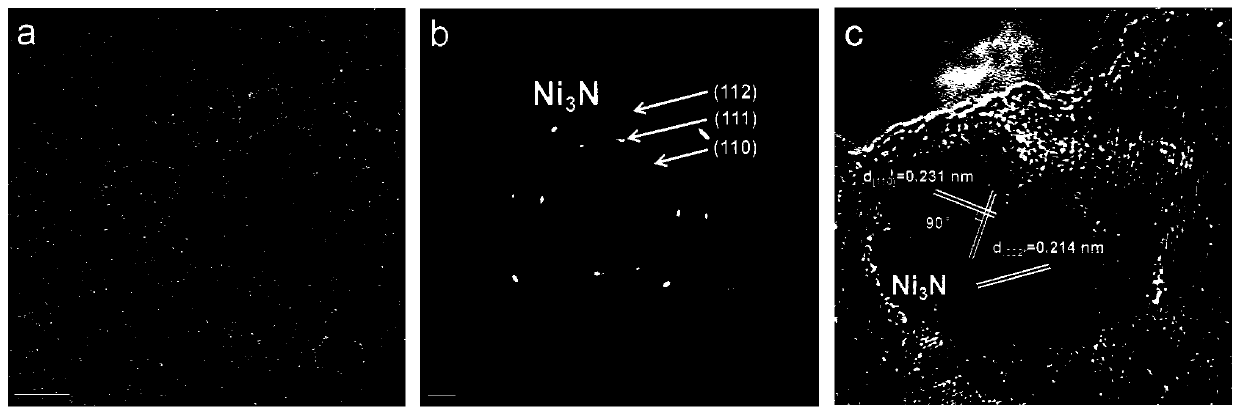
[0040] With nickel foam as the carrier, its thickness is 1.60mm, and its surface density is ~650g / m 2 , the aperture is 0.20 ~ 0.80mm. Nickel Foam (1×4cm 2 ) were ultrasonically cleaned with ethanol, hydrochloric acid solution (1M) and deionized water for 10 minutes, together with 36mL containing Ni(NO 3 ) 2 ·6H 2 The deionized aqueous solution of O (0.015M) and HMT (0.03M) was placed in a hydrothermal kettle with a volume of 50mL, and after being treated at 100°C for 10 hours, it was naturally cooled to room temperature, and the prepared sample was fully cleaned and then vacuumed at room temperature. Dry for 6 hours to obtain the hydrothermal sample Ni(OH) 2 / NF; hydrothermal sample in NH 3 / Ar atmosphere heated to 380 ° C, the heating rate of 5 ° C / min, after 2 hours of constant temperature heat treatment and then cooled to room temperature, the target catalyst Ni 3 N / Ni / NF.
[0041] The phase / structural characterization of the catalyst obtained in this embodiment: ...
Embodiment 2
[0051] With carbon cloth (CC, 1×4cm 2 ) as the carrier, after ultrasonic cleaning with hydrochloric acid (1M), absolute ethanol and deionized water for 20 minutes each, the carbon cloth was kept in concentrated nitric acid (0.5M) at 90°C for 4 hours, and deionized water and deionized ethanol After washing and drying, put it into a hydrothermal kettle equipped with a transition metal salt solution and a precipitating agent. The transition metal salts, precipitants and their concentrations used in the hydrothermal reaction process are: NiCl 2 ·6H 2 O (0.05M), HMT (0.1M), hydrothermal reaction condition is 140 ℃ constant temperature for 16 hours; hydrothermal state sample in NH 3 / Ar atmosphere was heated to 450° C., the heating rate was 10° C. / min, and after 9 hours of constant temperature heat treatment, it was cooled to room temperature to obtain the target catalyst.
[0052] The phase / structural characterization of the catalyst obtained in this embodiment:
[0053] (1) Th...
Embodiment 3
[0059] With nickel foam as the carrier, its thickness is 1.60mm, and its surface density is ~650g / m 2, the aperture is 0.20 ~ 0.80mm. Nickel Foam (1×4cm 2 ) were ultrasonically cleaned with ethanol, hydrochloric acid solution (1M) and deionized water for 10 minutes, together with 36 mL of Co(NO 3 ) 2 ·6H 2 The deionized aqueous solution of O (0.1M) and HMT (0.2M) was placed in a hydrothermal kettle with a volume of 50mL, and after being treated at 150°C for 5 hours, it was naturally cooled to room temperature. The prepared sample was fully cleaned and then vacuumed at room temperature. Dry for 6 hours to obtain the hydrothermal sample Co(OH) 2 / NF; hydrothermal sample in NH 3 / Ar atmosphere heated to 400 ° C, heating rate 5 ° C / min, after 2 hours of constant temperature heat treatment and then cooled to room temperature, the target catalyst Co 3 N / Co / NF.
[0060] Catalyst Co obtained in this embodiment 3 Electrocatalytic performance test of N / Co / NF:
[0061] The obt...
PUM
| Property | Measurement | Unit |
|---|---|---|
| particle size | aaaaa | aaaaa |
| size | aaaaa | aaaaa |
| thickness | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com