18F labeled compound and pod protease targeted PET imaging probe
A compound, 18F technology, applied in the field of radiopharmaceuticals and nuclear medicine, can solve the problem that positron emission tomography has not been applied, and achieve the effects of reducing non-specific uptake, increasing specificity, and increasing water solubility
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0073] 18 The F-labeled compound has a precursor compound of the structure shown in the following formula 1, and its synthetic route is,
[0074]
[0075] Intermediate compound 1-2 was synthesized according to the method reported in the literature [Lin, J. et al. Chem Commun (Camb) 2017, 53, (48), 6476-6479.],
[0076]
[0077] Weigh compound 1-2 (38mg, 0.08mmol), compound 1-1 (50mg, 0.09mmol) and HBTU (62mg, 0.16mmol), add it to the reaction bottle, and dissolve it with ultra-dry DMF ultrasonically for 1min (model: KQ2200E, power : 100W), then add DIPEA (88μL, 0.53mmol), N 2 Reaction at room temperature under protection for 2h. After separation and purification by column chromatography (eluent: DCM:MeOH (volume ratio) = 35:1 to 15:1, the solvent was collected and rotary evaporated, and dried in vacuum for 2 hours to obtain compound 1-3 (60 mg, yield 72%) .
[0078] The obtained compound 1-3 was dissolved in 4 mL of DCM:MeCN:TFA=1:1:2 (volume ratio), the solution tur...
Embodiment 2
[0083] 18 The F-labeled compound has a precursor compound of the structure shown in the following formula 2, and its synthetic route is,
[0084]
[0085] Polypeptide Ac-HEHEHEAAN-OH, compound 2-1, was synthesized by solid-phase peptide synthesis, and the synthetic route was as follows:
[0086] By sequentially linking Fmoc-N-trityl-L-asparagine (298 mg, 0.5 mmol), N-fluorenylmethoxycarbonyl-L-alanine (156 mg, 0.5 mmol), N-fluorenylmethoxycarbonyl- L-alanine (156mg, 0.5mmol), Fmoc-L-glutamic acid-5-tert-butyl ester hydrate (213mg, 0.5mmol), Fmoc-trityl-L-histidine (310mg, 0.5 mmol), Fmoc-L-glutamic acid-5-tert-butyl hydrate (213mg, 0.5mmol), Fmoc-trityl-L-histidine (310mg, 0.5mmol), Fmoc-L-glutamine Acid-5-tert-butyl hydrate (213mg, 0.5mmol), Fmoc-trityl-L-histidine (310mg, 0.5mmol), acetic anhydride (1mL, 10mmol), de-resin, rotary evaporation solvent, Ether was precipitated, and compound 2-1 was obtained after vacuum drying (634 mg, yield 56%).
[0087]
[0088] Com...
Embodiment 3
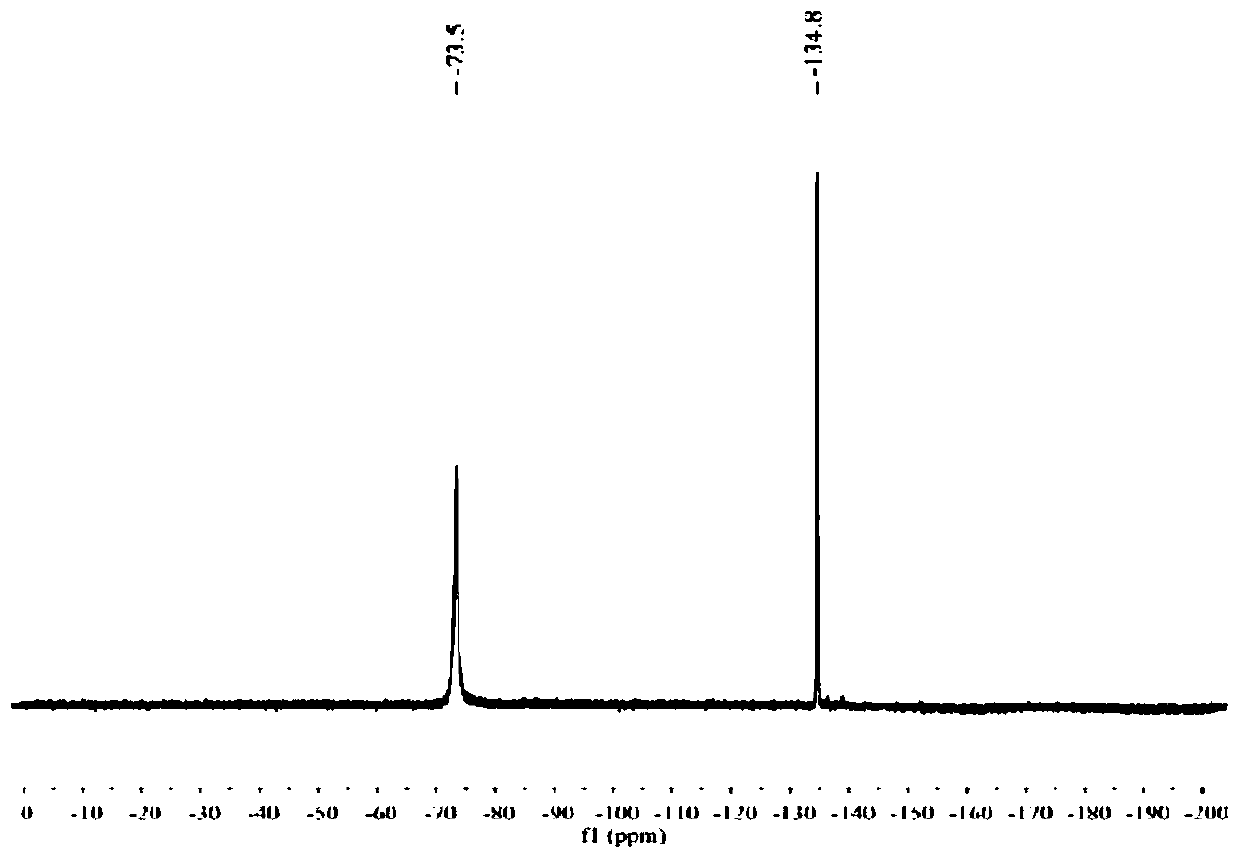
[0094] Radiosynthesis:
[0095] probe 18 The radiosynthetic route of F-1 compound is as follows,
[0096]
[0097] Produced on demand by a medical cyclotron 18 F fluoride ion. After production is complete, the 18 The F fluoride ion is passed to the anion exchange column (QMA), and the pyridazine buffer solution (300 μL, pH=2.5) is used to 18 The F anion (150-300mCi) is eluted from the QMA column into a polypropylene reaction tube (1mL), and the AmBF synthesized in Example 1 3 -CBT-Cys(StBu)-AAN (precursor compound of the structure shown in formula 1, that is, probe precursor 1) (25mM, 30μL) was added to the reaction tube and incubated at 80°C for 30min (80°C is the radiosynthesized Optimal conditions).
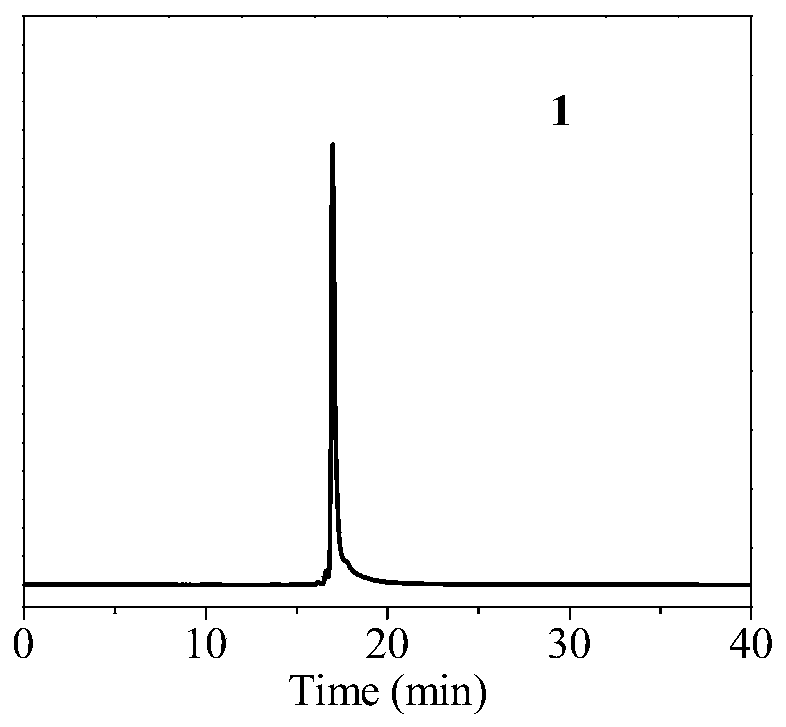
[0098] Radioactive purification:
[0099] After the radiosynthesis is complete, transfer the reaction solution to a centrifuge tube containing 20 mL of ultrapure water, and then the resulting radiolabeled probe 18 F-1 was successively loaded on a C18 purification co...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com