Method for promoting secretory expression of corn gibberelenone degrading enzyme ZHD 518 protein as well as application
A technology of ZHD518 and zearalenone, applied in the field of molecular biology, can solve the problem of low expression of ZHD518, optimize the conditions for inducing expression, simplify the steps of protein separation and purification, and reduce waste.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0050] Example 1: Construction of expression vectors in which three fusion signal peptides (PhoA, OmpA, PelB) and ZHD 518 gene are connected in series in Escherichia coli BL21 (DE3) (taking PhoA as an example)
[0051] (1) The PhoA-ZHD 518 fragment was amplified by three rounds of polymerase chain reaction (PCR). The first round of PCR amplification obtained the ZHD 518 gene fragment, the second round of PCR amplification obtained the PhoA signal peptide gene fragment, and the third round connected the ZHD 518 gene fragment and the PhoA signal peptide gene fragment into a large fragment ( PhoA-ZHD 518). Among them, the three rounds of PCR system are the same, and the amplification program varies slightly with the length of the fragment.
[0052] PCR reaction system: 10×Pfubuffer, 5 μL; dNTP (2.5mM), 5 μL; primer F (10 μM), 2 μl; primer R (10 μM), 2 μL; Pfu polymerase, 0.5 μL; Synthetic), 1 μL; add ddH 2 O to 50 μL of the reaction system.
[0053] PCR amplification program:...
Embodiment 2
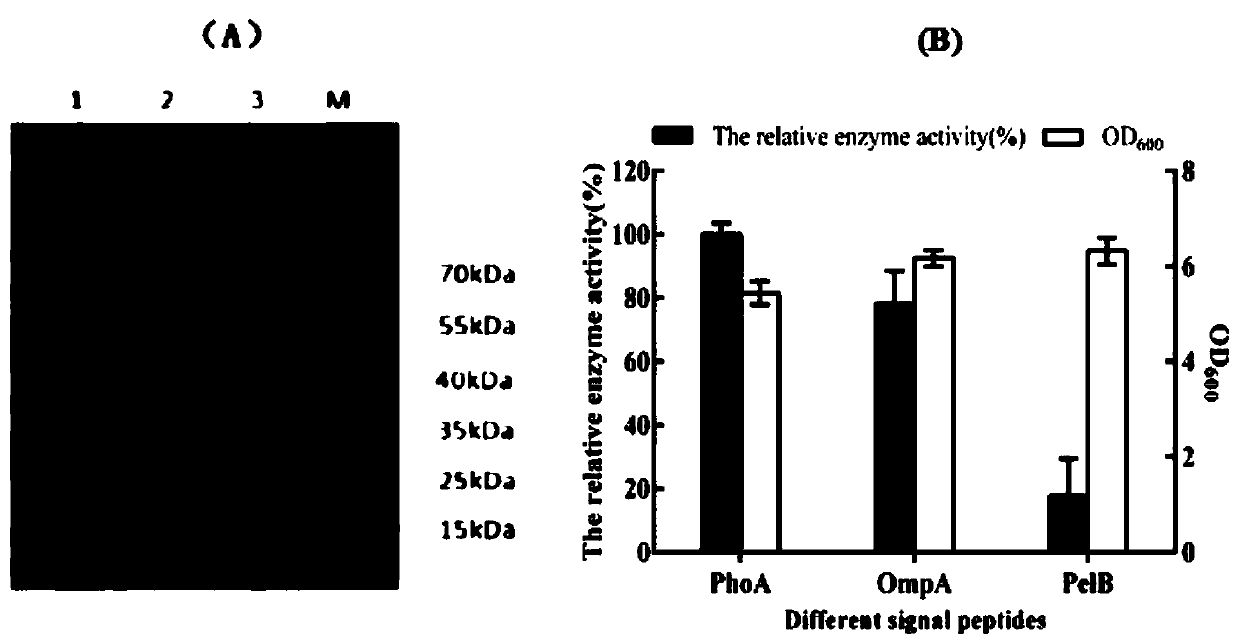
[0065] Example 2: Induced expression of ZHD 518 protein containing fusion signal peptide in Escherichia coli
[0066] The fusion expression vectors pOmpA-ZHD 518, pPelB-ZHD 518, and pPhoA-ZHD518 constructed in Example 1 were respectively transformed into Escherichia coli BL21 (DE3) competent cells. Transformation system: 1 μL of recombinant plasmid; 10 μL of competent cells; after mixing the system, place it on ice for 10 minutes, then bathe in water at 42°C for 35 seconds, add 150 μL of LB medium, incubate on a shaker at 37°C for 1 hour, and then spread it on the Incubate on plain LB plates at 37°C for 12-16h.
[0067] Inoculate a single colony into 20mL liquid LB medium, add 50μg / mL kanamycin, incubate at 37°C for 12-16h, transfer to 50mL liquid LB medium according to the inoculum size of 1%, add 50μg / mL kanamycin Mycin, cultured at 37°C, until OD 600 When reaching 1.2-1.5, add IPTG with a final concentration of 0.5mM, and then transfer to 28°C for low temperature inductio...
Embodiment 3
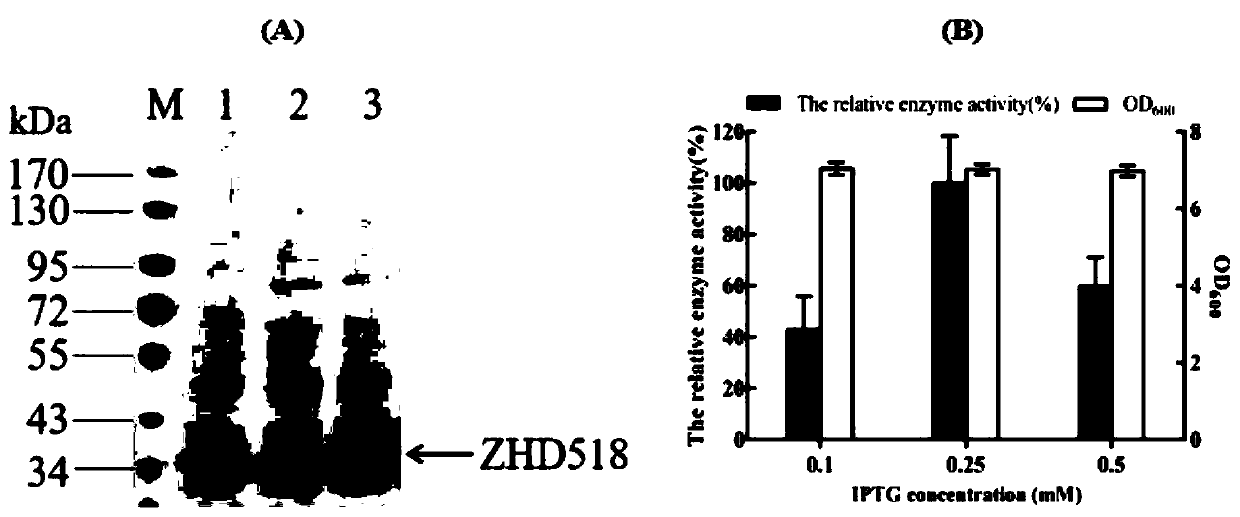
[0069] Example 3: Optimization of the induced expression conditions of the ZHD 518 protein containing the fusion signal peptide in Escherichia coli
[0070] The fusion expression vector OmpA-ZHD 518 with the best expression in Example 2 was optimized for secretion conditions, and a single colony was picked from the LB plate coated in Example 2 and inoculated in 20 mL of liquid TB medium, and 50 μg / mL of Kanamycin, cultured at 37°C for 12-16h, transferred to 50mL liquid TB medium according to the inoculum size of 1%, added 50μg / mL kanamycin, cultured at 37°C, waited for OD 600 When it reaches 1.2-1.5, after adding different final concentrations of IPTG, place the shaker flask in a shaker with a temperature of 28°C to induce expression and take 28h of fermentation broth to react with the substrate to determine the relative enzyme activity. The specific method is consistent with Example 2 , the result is as image 3 A and 3B.
[0071] The fusion expression vector OmpA-ZHD 518 w...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com