Method for detecting gypenoside A in gelan Xinning soft capsules by adopting HPLC-UV method
A technology of Gypenoside and Gypenoside, which is applied in the direction of measuring devices, instruments, scientific instruments, etc., can solve the problems of poor accuracy and cumbersome operation process, and achieve good durability, simple processing and detection process, and good accuracy Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
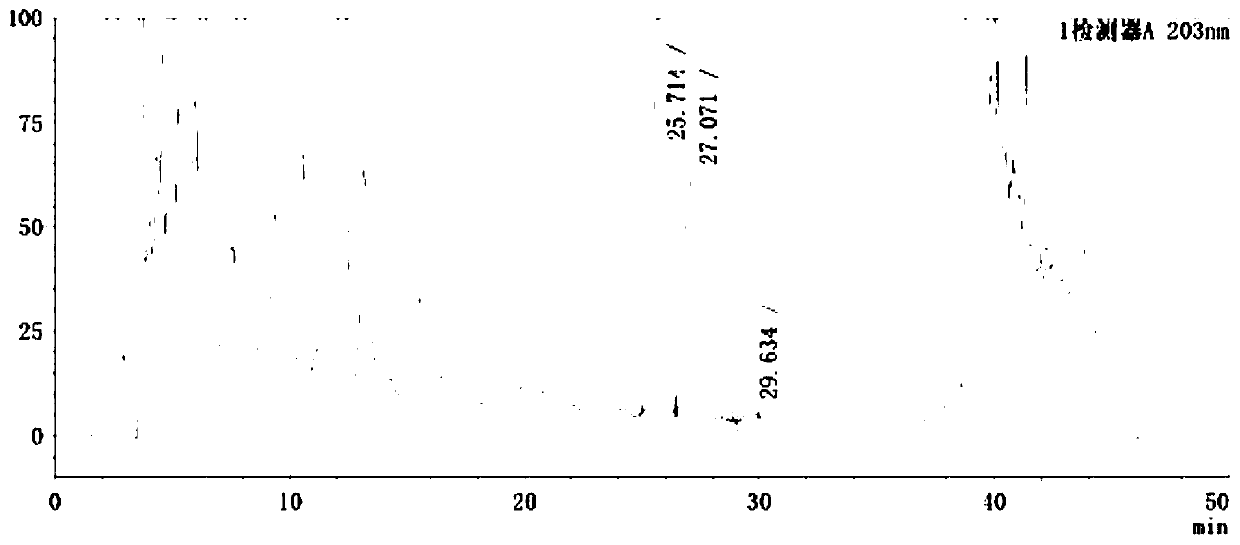
[0035] Embodiment 1: A kind of detection method adopting HPLC-UV method to measure gypenosides in Gelanxinning soft capsule comprises the following steps:
[0036] Step 1: Preparation of GLAXINING Soft Capsules and Negative Control Capsules
[0037] 1. Preparation of Granxinine Soft Capsules
[0038] Gelanxinning soft capsule prescription: 200g total flavonoids of kudzu root, 60g hawthorn extract, 20g gypenosides, 254g salad oil, 6g beeswax, 20g hydrogenated palm oil, 10g soybean lecithin, 0.5g methyl silicone oil, made into 1000 capsules.
[0039] Preparation method of Gelanxinning soft capsule: take salad oil, add beeswax and hydrogenated palm oil, heat to dissolve, let cool, add soybean lecithin, stir well, add kudzu root flavonoids, hawthorn extract, gypenosides, stir well, Grind the colloid evenly, add methyl silicone oil, decompress and defoam, and press the medicine to make soft capsules.
[0040] 2. Preparation of Negative Control Capsules
[0041] Gypenosides were ...
Embodiment 2
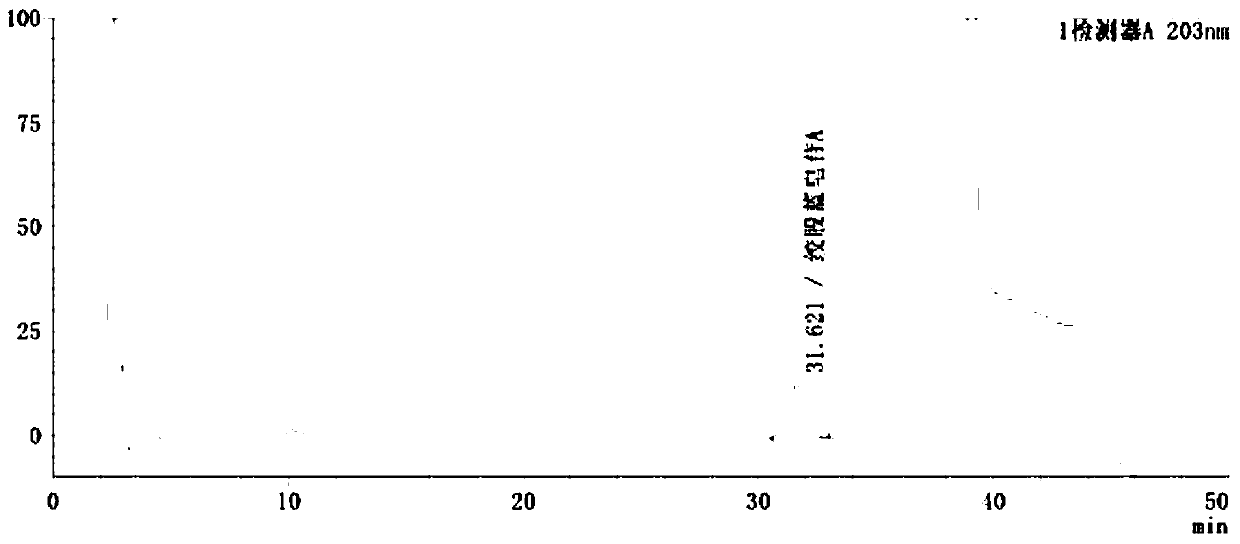
[0115] Embodiment 2: A method for detecting gypenoside A in Gelanxinning soft capsules by HPLC-UV method, characterized in that: after Gelanxinning soft capsules are pretreated, high performance liquid chromatography (HPLC- UV) measurement, that is, each Gelanxinning soft capsule contains gypenosides and gypenoside A (C 46 h 74 o 17 ), not less than 0.5mg, the specific steps are as follows:
[0116] (1) Preparation of the test solution: take 8 capsules of Glacicin soft capsules, add 90ml of 10% methanol, weigh them, reflux in a water bath for 1 hour, let cool, weigh again, add 10% methanol to reduce the weight. Lost weight, shake well, centrifuge;
[0117] After centrifugation, take the supernatant, discard the oil layer, weigh 23ml of the water layer, put it in a separatory funnel, shake and extract once with 23ml of methyl tert-butyl ether, discard the ether solution, and shake the water solution with n-butanol saturated with water. Shake and extract 3 times, 23ml each t...
Embodiment 3
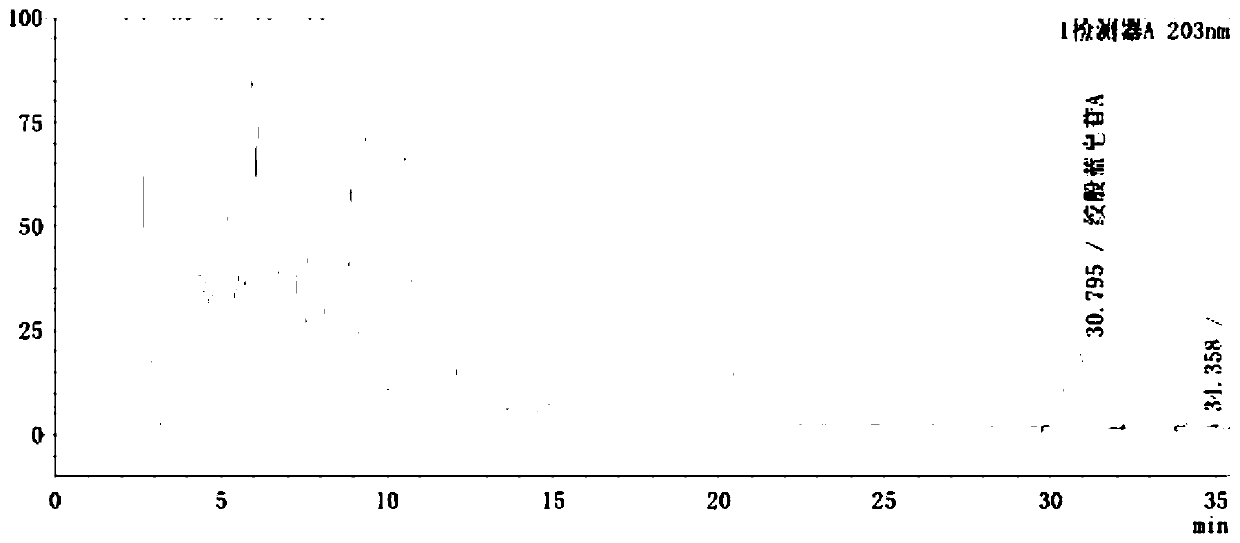
[0122] Embodiment 3: a kind of method adopting HPLC-UV method to detect gypenoside A in Gelanxinning soft capsule, it is characterized in that: after Gelanxinning soft capsule is pretreated, directly adopt high-performance liquid chromatography (HPLC- UV) measurement, that is, each Gelanxinning soft capsule contains gypenosides and gypenoside A (C 46 h 74 o 17 ), not less than 0.5mg, the specific steps are as follows:
[0123] (1) The preparation of need testing solution: get Glandine soft capsule: 11, add 10% methyl alcohol 110ml, weigh, water-bath reflux, let cool, weigh again, supplement lost with 10% methanol weight, shake, centrifuge;
[0124] After centrifugation, take the supernatant, discard the oil layer, weigh 27ml of the water layer, put it in a separatory funnel, shake and extract twice with 27ml of methyl tert-butyl ether, discard the ether solution, and shake the water solution with n-butanol saturated with water. Shake and extract 5 times, 27ml each time, co...
PUM
| Property | Measurement | Unit |
|---|---|---|
| recovery rate | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com