MALDI-TOF-MS based high-throughput analysis method for single-particle capsule drug
A technology of MALDI-TOF-MS and analysis method, which is applied in the direction of material analysis, analysis materials, and measuring devices through electromagnetic means, which can solve the problems of unused drug main components and achieve drug screening and high-throughput Effects of Drug Screening
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
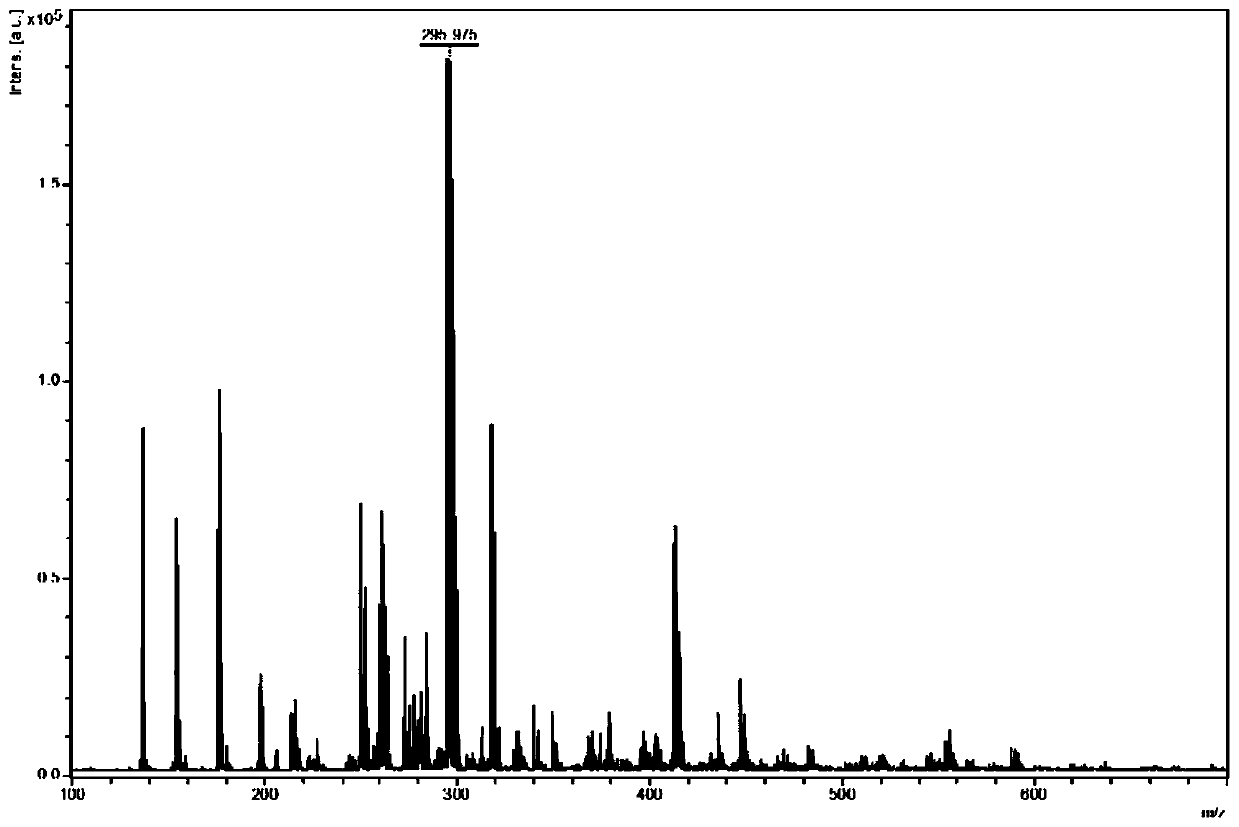
[0038] The MALDI-TOF-MS analysis method of active pharmaceutical ingredient in diclofenac sodium capsule, comprises the following steps:
[0039] (1) Instruments and reagents
[0040] The mass spectrometer used in the embodiment of the present invention is Bruker rapifleX MALDI Tissuetyper system; the balance used is Mettler Toledo XS 105 electronic balance.
[0041] The reagents used are chromatographically pure acetonitrile, methanol and ethanol from Merck, Germany; the standard used is the chromatographic standard product of diclofenac sodium from Chengdu Durst Biotechnology Co., Ltd.; the matrix used is 2,5-dihydroxybenzene from Sigma. Formic acid (DHB) and alpha-cyano-4-hydroxycinnamic acid (CHCA) matrix.
[0042] The drug samples were collected from a pharmaceutical factory in Nanjing, and the drug samples were in granular form.
[0043] (2) Sample preparation
[0044] The diclofenac sodium standard substance was made into a standard solution with a concentration of 1...
Embodiment 2
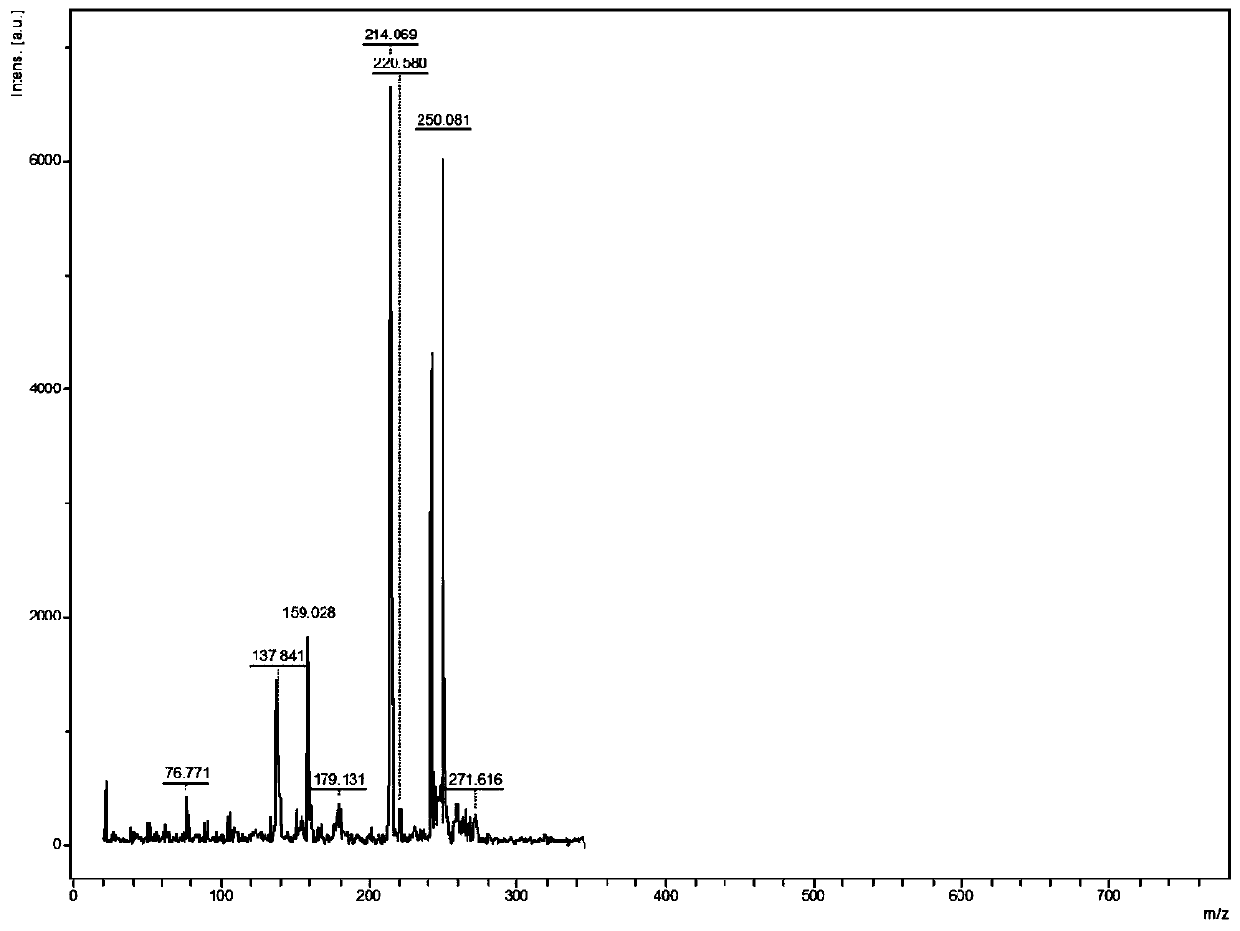
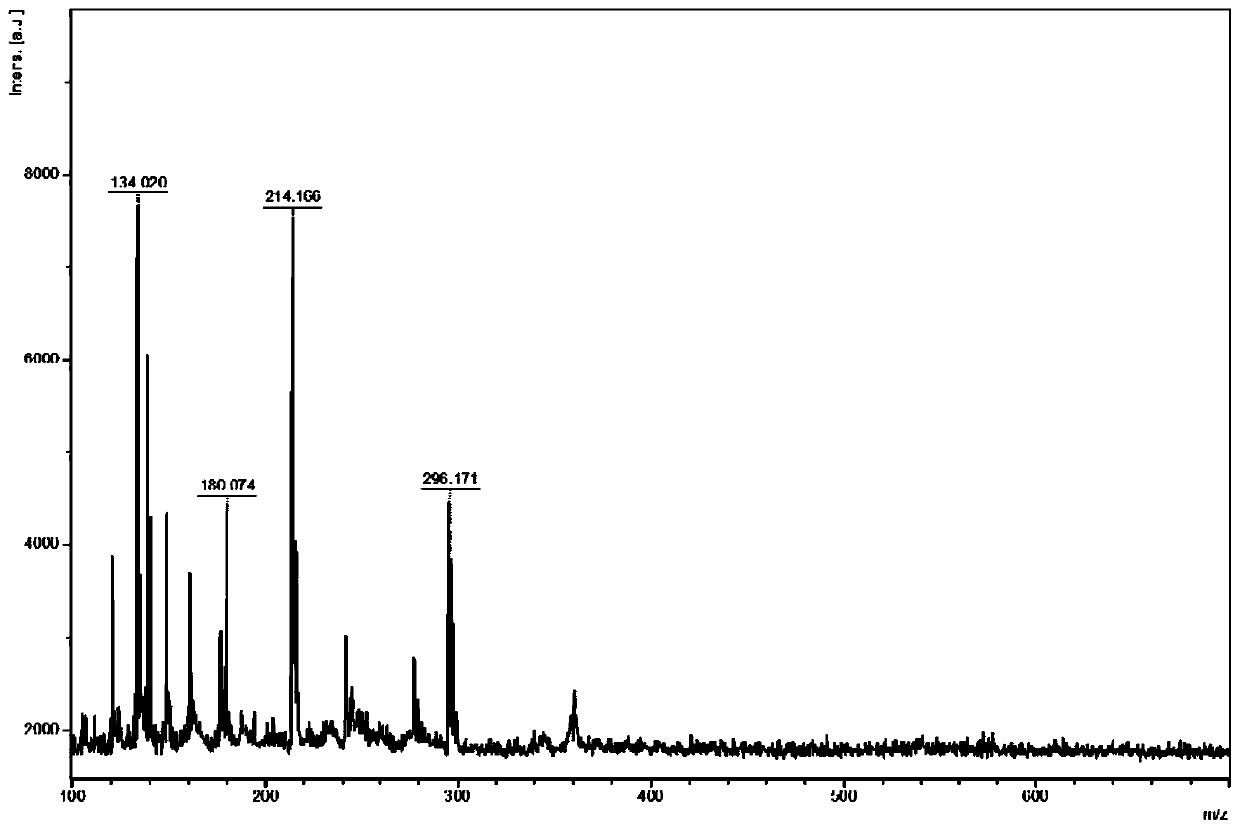
[0065] The MALDI-TOF-MS analysis method of active pharmaceutical ingredients in itraconazole capsules comprises the following steps:
[0066] (1) Instruments and reagents
[0067] The mass spectrometer used in the embodiment of the present invention is Bruker rapifleX MALDI Tissuetyper system; the balance used is Mettler Toledo XS 105 electronic balance.
[0068] The reagents used were chromatographically pure acetonitrile, methanol and ethanol from Merck, Germany; the standard used was itraconazole chromatographic standard from Chengdu Durst Biotechnology Co., Ltd.; the matrix used was DHB and CHCA from Sigma.
[0069] The drug samples were collected from a pharmaceutical factory in Chengdu, and the drug samples were in granular form.
[0070] (2) Sample preparation
[0071] Acetonitrile, methanol and ethanol were used to prepare standard itraconazole standard solutions with a concentration of 1.0 mg / mL.
[0072] DHB and CHCA were respectively prepared with methanol / water ...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com