Novel polyamide polymer material based on 2,5-diallyloxy p-phenylenediamine monomer and preparation method of novel polyamide polymer material
A technology of diallyloxy-p-phenylenediamine and diallyloxydiamino, which is applied in the field of new polyamide polymer materials and its preparation, and can solve the problems of limited compatibility, high crystallinity, and surface chemical activity. Low-level problems, to achieve the effect of improving the interface bonding strength, good interface recombination, and excellent thermal stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
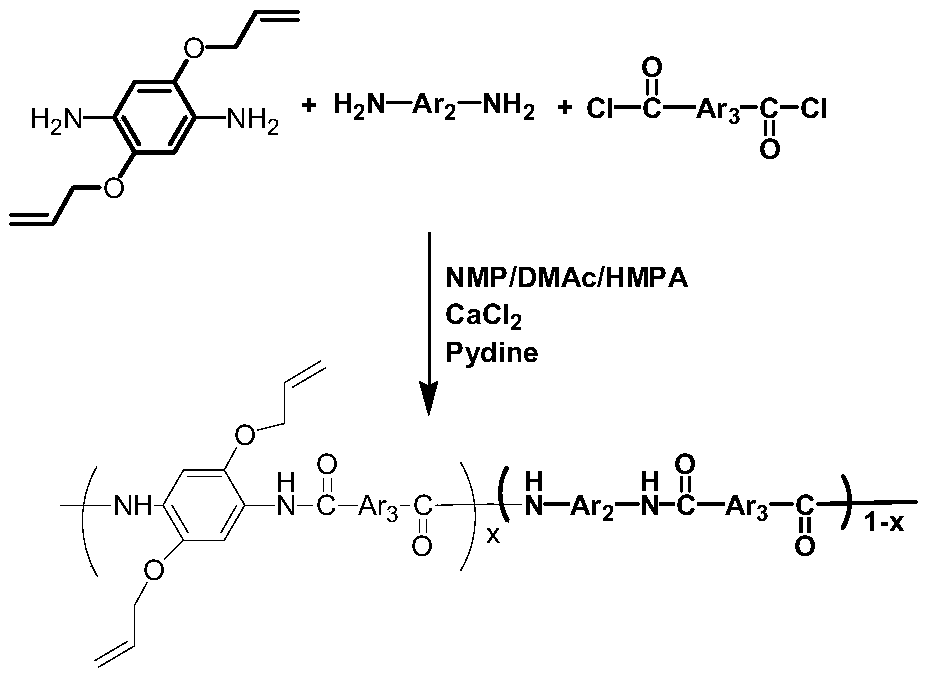
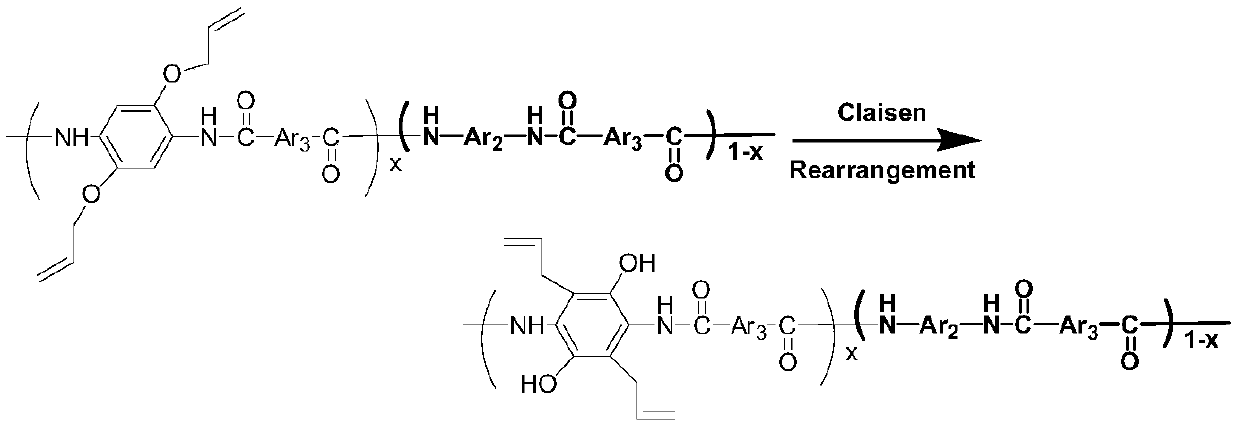
[0068] In this embodiment, the novel polyarylamide resin material containing allyloxy groups has the following repeating structural units:
[0069]
[0070] The specific preparation method is as follows: when equipped with stirring paddle, drying tube and N 2 Add 50ml of solvent N-methylpyrrolidone, cosolvent anhydrous CaCl 2 4.12g, add 12.48mmol (1.3496g) of diamino monomer p-phenylenediamine and 4.16mmol (0.9163g) of 2,5-diallyloxy-p-phenylenediamine successively, stir to dissolve them all, and put the mixture in- Cool at 10°C for 15min, under the conditions of -10°C and rapid stirring, add 16.64mmol (3.3793g) of terephthaloyl chloride and 29.95mmol (2.4ml) of pyridine in two times, and accelerate the stirring, preferably without splashing . Keep the temperature of the system at -10°C and stir for 0.5h of polycondensation reaction, then continue to stir at 30°C for 6h of polycondensation reaction, pour the mixture into a large amount of water for precipitation, wash wit...
Embodiment 2
[0074] In this embodiment, the novel polyarylamide resin material containing allyloxy groups has the following repeating structural units:
[0075]
[0076] The specific preparation method is as follows: when equipped with stirring paddle, drying tube and N 2 Add 50ml of solvent N-methylpyrrolidone, cosolvent anhydrous CaCl 2 4.12g, add 8.32mmol (0.8997g) of diamino monomer p-phenylenediamine and 8.32mmol (1.8326g) of 2,5-diallyloxy-p-phenylenediamine successively, stir to dissolve them all, and put the mixture in- Cool at 10°C for 15min, under the conditions of -10°C and rapid stirring, add 16.64mmol (3.3793g) of terephthaloyl chloride and 29.95mmol (2.4ml) of pyridine in two times, and accelerate the stirring, preferably without splashing . Keep the temperature of the system at -10°C and stir for 0.5h of polycondensation reaction, then continue to stir at 30°C for 6h of polycondensation reaction, pour the mixture into a large amount of water for precipitation, wash with...
Embodiment 3
[0080] In this embodiment, the novel polyarylamide resin material containing allyloxy groups has the following repeating structural units:
[0081]
[0082] The specific preparation method is as follows: when equipped with stirring paddle, drying tube and N 2 Add 50ml of solvent N-methylpyrrolidone, cosolvent anhydrous CaCl 2 4.12g, successively add 4.16mmol (0.4499g) of diamino monomer p-phenylenediamine, 12.48mmol (2.7490g) of 2,5-diallyloxy p-phenylenediamine, stir to dissolve them all, and the mixture is placed in - Cool at 10°C for 15min, under the conditions of -10°C and rapid stirring, add 16.64mmol (3.3793g) of terephthaloyl chloride and 29.95mmol (2.4ml) of pyridine in two times, and accelerate the stirring, preferably without splashing . Keep the temperature of the system at -10°C and stir for 0.5h of polycondensation reaction, then continue to stir at 30°C for 6h of polycondensation reaction, pour the mixture into a large amount of water for precipitation, wash...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com