Novel antibacterial lipopeptide compound and preparation method and application thereof
A technology of compound and lipopeptide, which is applied in the field of preparation of the lipopeptide compound, can solve the problem of little effect on drug-resistant pathogenic bacteria
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
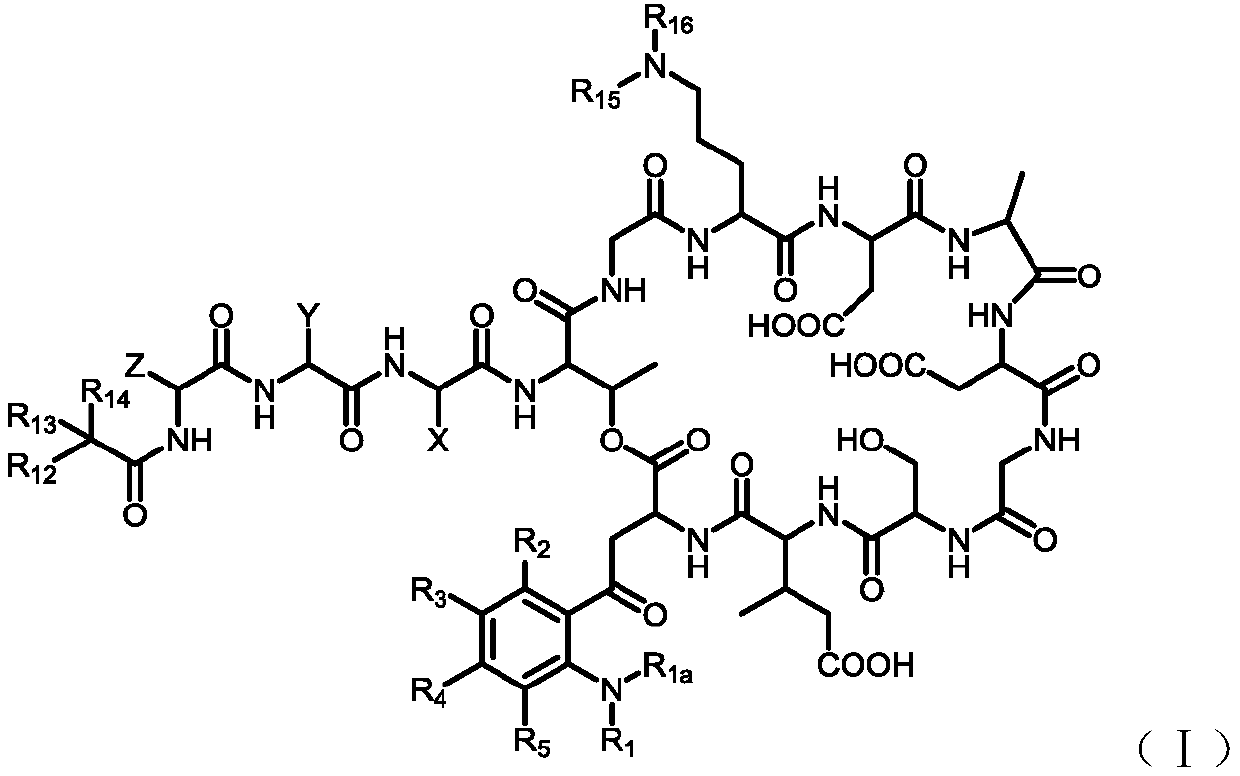
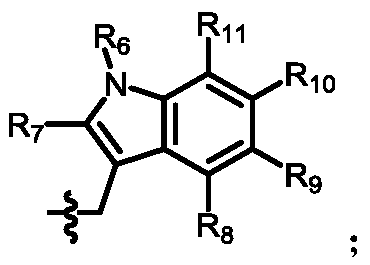
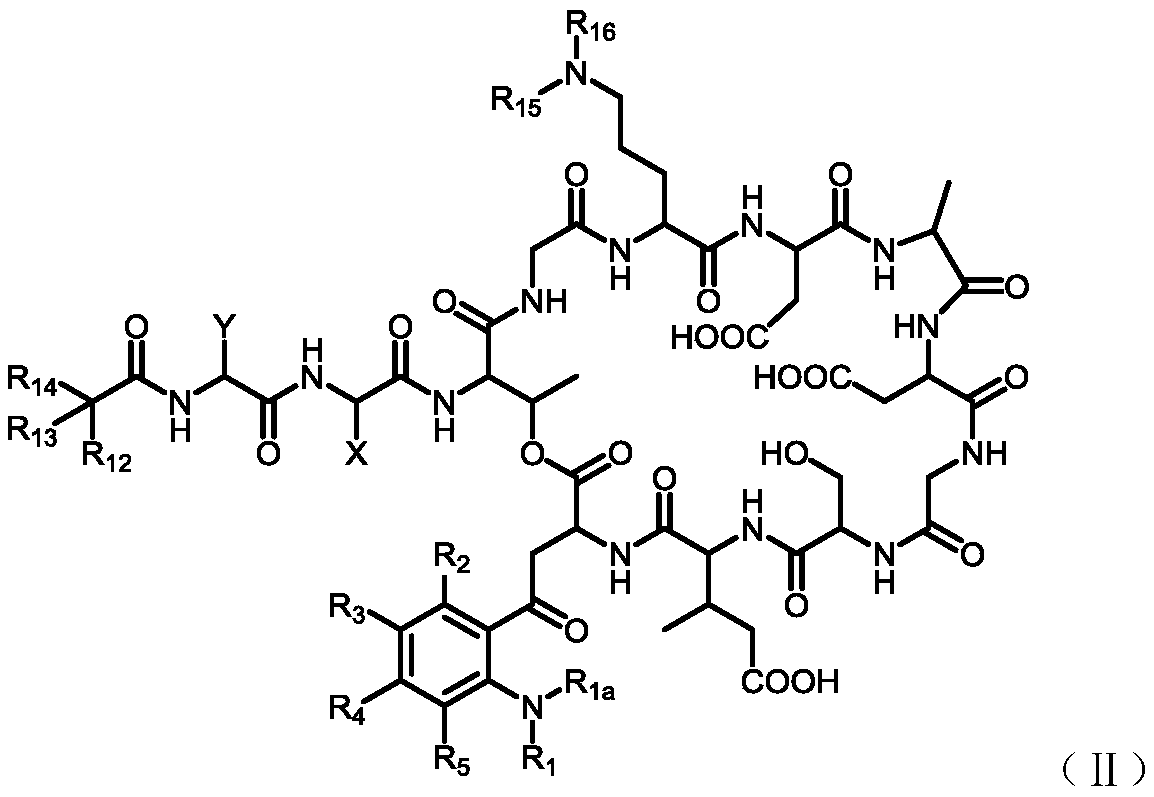
[0151] 4. Preparation of intermediate (Inter-I)
[0152] The preparation of the intermediate (Inter-I) can start from daptomycin, first with di-tert-butyl dicarbonate, (Boc) 2 O reacts to add a tert-butoxycarbonyl protecting group (Boc) to the amino group of ornithine , The amino group on the aniline is then alkylated using a reductive amination reaction. Reagents for reductive amination can be an aldehyde plus sodium cyanoborohydride. Acetaldehyde, formaldehyde, and propionaldehyde are used in these reactions to convert anilines to phNHEt, phNHMe, and phNHPr groups, respectively.
[0153] Specifically, daptomycin (1) reacts with di-tert-butyl dicarbonate and triethylamine, and a tert-butoxycarbonyl protecting group (Boc) is added to the amino group of ornithine to generate daptomycin-Boc (2 ).
[0154]
[0155]
[0156] Daptomycin-Boc(2) reacts with formaldehyde, acetaldehyde or propionaldehyde in the presence of sodium cyanoborohydride at room temperature to perfor...
Embodiment 1
[0205] The preparation of embodiment 1 Inter-IA
[0206]
[0207] Step 1. Boc protection of ornithine amino group to generate daptomycin-Boc
[0208] A mixture of daptomycin (1.0 g) and water (6 mL) was stirred at room temperature for 1 hour and the solid gradually dissolved. To this solution was added triethylamine (600 uL) followed by a solution of di-tert-butyl dicarbonate (500 mg) in dimethyl sulfoxide (DMSO, 2 mL). The resulting suspension became a pale yellow solution after being vigorously stirred for 1 hour, and liquid-mass analysis showed that daptomycin was completely converted into daptomycin-Boc. Positive-order ESIMS: m / z 1720.7(MH)+, with daptomycin-Boc(C 77 h 110 N 17 O28) was consistent with theoretical mass (Scheme A2). The reaction mixture was extracted with ethyl acetate (5 mL) to remove unreacted reagents, the aqueous layer was acidified with acetic acid (800 uL), then extracted twice with n-butanol (2 x 5 mL). The combined n-butanol solutions were ...
Embodiment 2
[0211] The preparation of embodiment 2 Inter-IB
[0212]
[0213] To a solution of daptomycin-Boc (500 mg) in methanol (5 mL) was added acetic acid (360 uL), followed by 37% aqueous formaldehyde (900 uL). The resulting mixture was stirred at room temperature for 5 minutes, then mixed with a solution of sodium cyanoborohydride (360 mg) in methanol (2 mL). The reaction solution was stirred at room temperature for 20 minutes and the product was purified by HPLC (Scheme B1). The peak at 16 minutes was collected and evaporated in vacuo to give Inter-IB as a yellow powder (255 mg). Negative polarity ESIMS: m / z1732.6(M-H) - , with Inter-IB(C 78 h 110 N 17 o 28 ) are consistent with the theoretical mass (Scheme A1-Neg).
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com