Application of depsidone compound in preparation of neuroprotective drugs
A technology of depsipphenolic acid cyclic ether and neuroprotection, which is applied in the field of biochemical medicine and can solve the problems of high toxicity and side effects and high price
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
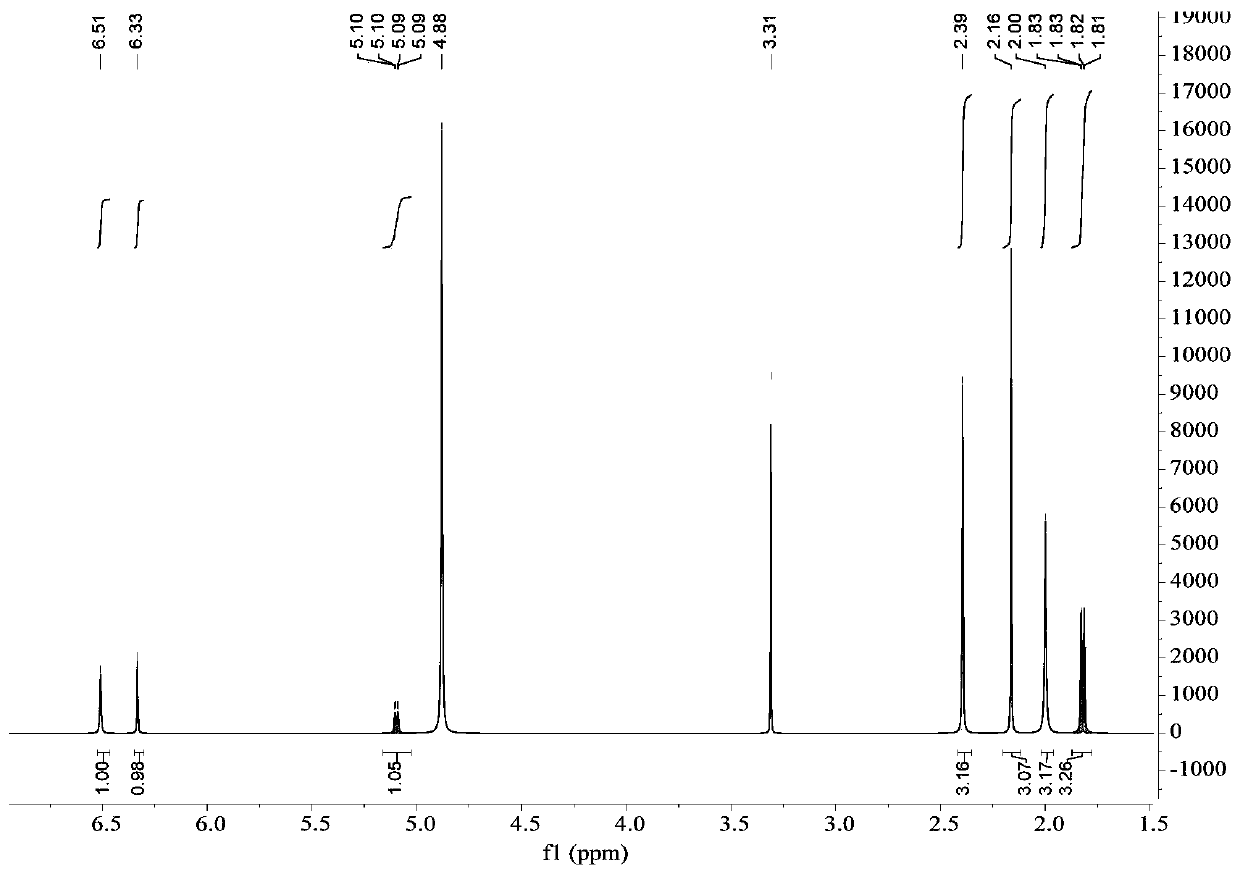
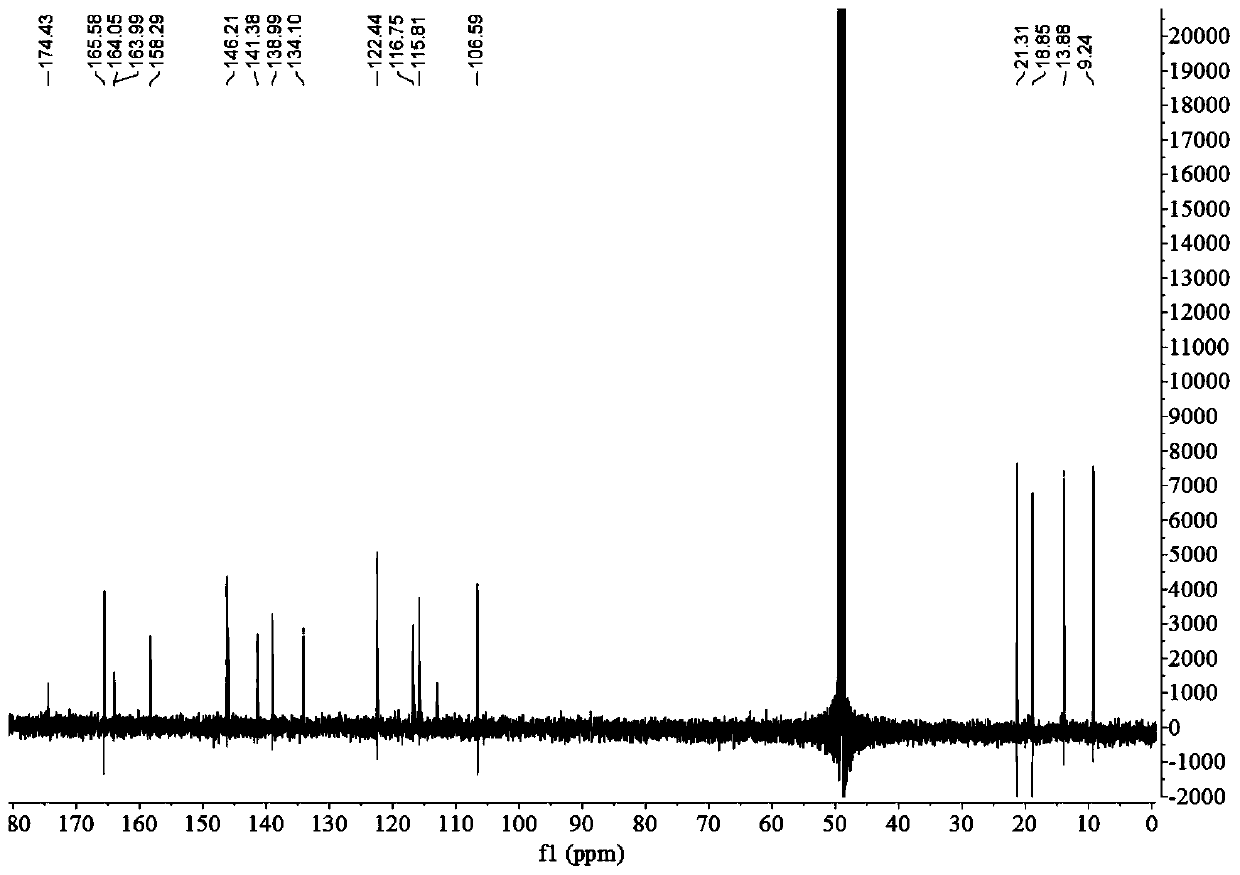
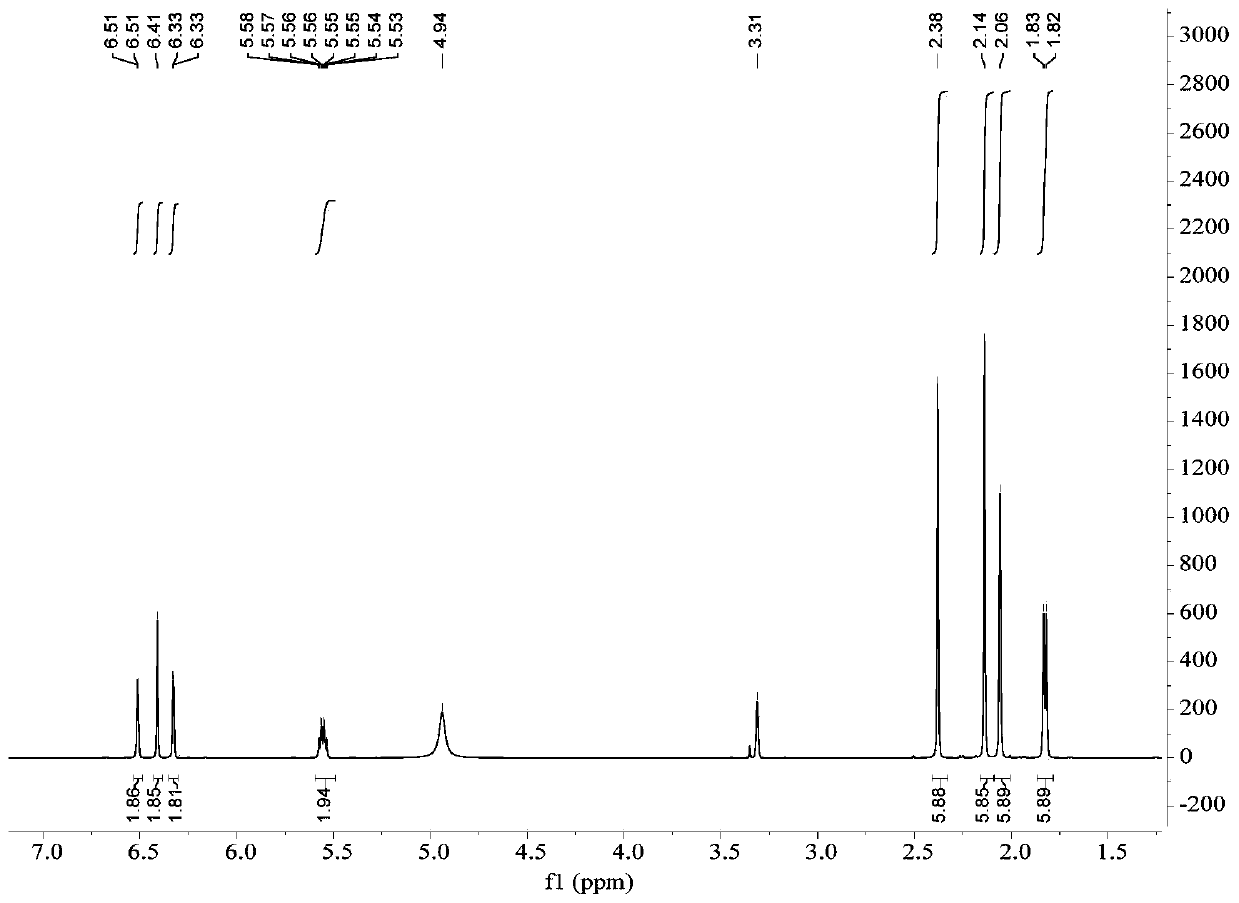
Image
Examples
Embodiment 1
[0054] The preparation of embodiment 1 depsipphenolic acid cyclic ether compound
[0055] The preparation method of depsipphenolic acid cyclic ether compound comprises the following steps:
[0056] S1. Fermentation: inoculate the marine fungus Aspergillus unguis 6-20-6 in the fungal liquid culture medium and leave it to ferment for 21 days, filter, and collect the mycelia and fermentation broth respectively;
[0057] Wherein, the formula of the fungal liquid medium in step S1 is: 500 mL of fresh potato juice, 20 g of sea salt, 20 g of sucrose, 5.5 g of peptone, 500 mL of distilled water, and pH 7.
[0058] S2. Rough extraction: the fermentation broth obtained in step S1 is ultrasonically extracted 3 times with ethyl acetate and concentrated, and the mycelium obtained in step S1 is ultrasonically extracted 3 times with a mixed solvent of acetone and methanol and concentrated under reduced pressure, and the resulting concentration is The things are combined to obtain the crude ...
Embodiment 2
[0066] The determination of the neuroprotective activity of embodiment 2 depsipphenolic acid cyclic ether compounds
[0067] 1. Experimental method
[0068] After anesthetizing the pregnant mouse (ICR) at embryonic day 14 (E14), take out the embryo, separate the cerebral cortex, remove the outer dura mater, cut up the tissue, and separate the cerebral cortex nerve cells through trypsinization and other processes, suspend in the containing In 12% horse serum, 0.6% D-glucose and 2mM L-glutamine in neural basal medium (GibcoBRL), the neural cells were seeded in 96-well plates pretreated with 5 μg / mL poly-D-lysine (Sigma) , 37°C, 5% CO 2 Cultured under the condition of 20000 cells per well, the culture medium was replaced with 2% B-27 neural basal medium (Gibco BRL) after 3-12 hours.
[0069] Then, after the nerve cells were cultured in the B-27 medium for 4 days, different concentrations (1 μM, 10 μM) of the depsipaldehyde cyclic ether compounds Aspergillusidone A (group A1, gr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com