Preparation method of double-stimulation response type polyurethane micelle
A dual-stimuli-responsive, polyurethane technology, applied in the field of environment-responsive polymer material preparation, to achieve good pH response characteristics and temperature-sensitive characteristics, excellent biocompatibility and biodegradability effects
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
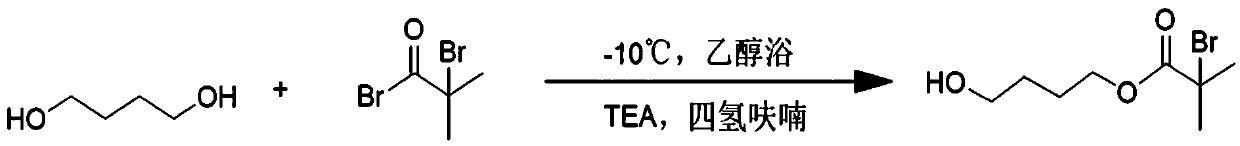
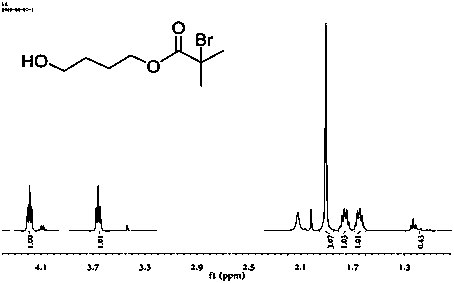
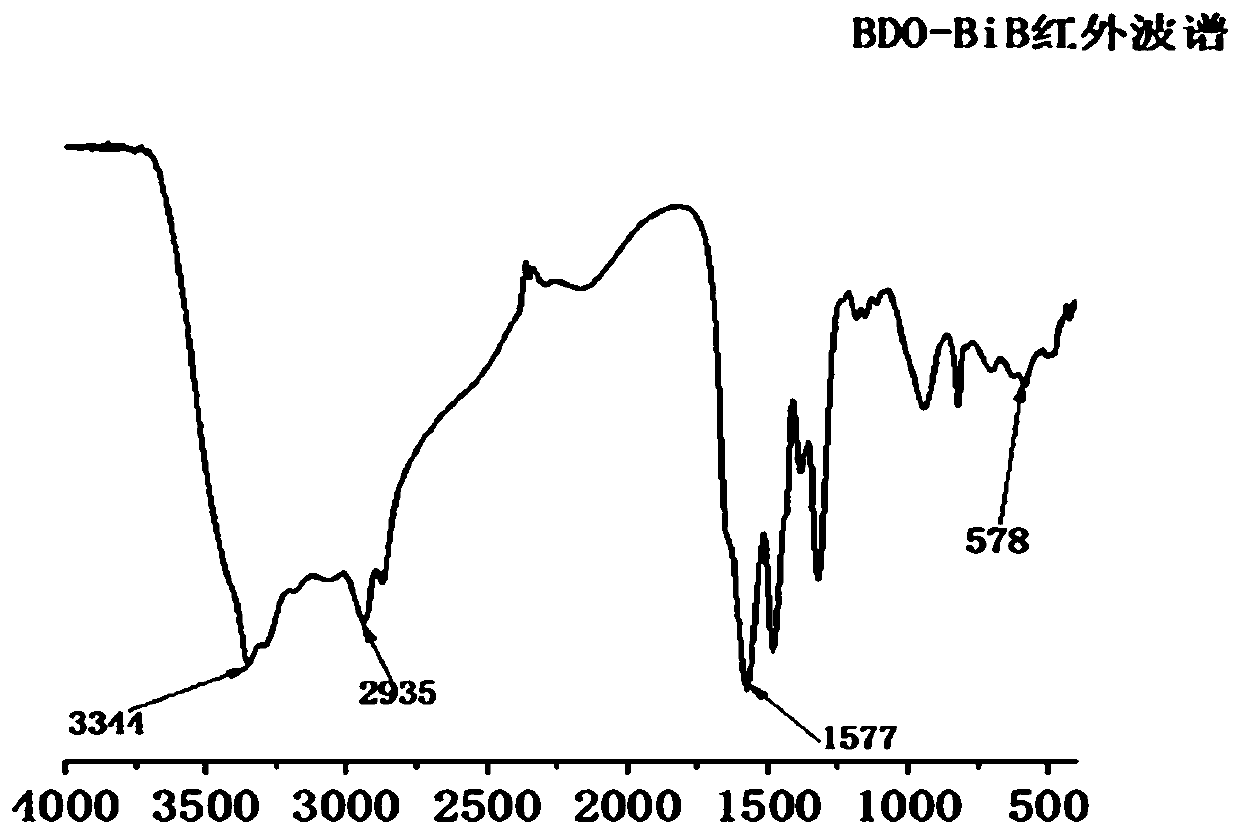
[0043] The preparation of embodiment 1 initiator
[0044] 1) Dissolve 0.3 mol of 1,4-butanediol (BDO) and 5 mL of triethylamine (TEA) in 40 mL of tetrahydrofuran, place in an ethanol bath at -10°C and stir for 10 minutes to obtain solution A.
[0045] 2) Dissolve 0.01 mol of 2-bromoisobutyryl bromide (BIBB) in 10 mL of tetrahydrofuran and place it in an ethanol bath at -10°C for 3 minutes; obtain solution B.
[0046] Then slowly drop the solution B into the solution A, and control the completion time of the dropping at about 30 minutes, and stir for 12 hours to react.
[0047] 3) After the reaction, the precipitate was removed by filtration, and the filtrate was rotary evaporated at 40°C to remove THF to obtain a light yellow liquid.
[0048] 4) Use CH 2 Cl 2 Extract light yellow liquid, wash three times with distilled water, anhydrous MgSO 4 Dry and rotary evaporate at 40°C to obtain the crude product as light yellow liquid.
[0049] 5) The crude product was separated an...
Embodiment 2
[0050] The preparation of embodiment 2 capping agent (HO-PDMA)
[0051] Prepare a capping agent with a molecular weight of 9000:
[0052] 1) Dissolve 0.0008 mol BDO-BiB, 0.05 mol monomer dimethylaminoethyl methacrylate (DMAEMA) and 0.0008 mol ligand methyldiethylenetriamine (PMDETA) in 10 mL N,N-dimethylformamide (DMF) under nitrogen gas and stirred at room temperature for 30 min.
[0053] 2) Add 0.0008 mol cuprous bromide (CuBr) into the reaction solution, continue to flow nitrogen and stir for 15 minutes.
[0054] 3) Stir and react the above stirred reaction solution in an oil bath at 50°C for 8 hours.
[0055] 4) After the reaction was completed, 10 mLTHF and 10 drops of methanol were added to the obtained reaction liquid, and it became clear.
[0056] 5) The reaction solution was separated by column chromatography to remove copper ions in the reaction solution, and the reaction solution was separated with THF and CH 3 The mixed solution of OH with a volume ratio of 20:...
Embodiment 3
[0062] The preparation of embodiment 3 vanillin acetal compound
[0063] 1) Mix 12 mmol vanillin, 15.6 mmol trimethylolethane and 0.12 mmol catalyst p-toluenesulfonic acid hydrate ( p -TsOH) was dissolved in 15 mL tetrahydrofuran (THF), and stirred at 90 °C under reflux for 12 h.
[0064] 2) After the reaction, THF was removed by rotary evaporation at 40°C to obtain a concentrated crude product.
[0065] 3) Cool the concentrated solution to room temperature, and carry out column chromatography separation and purification. The eluent used is a mixture of petroleum ether: ethyl acetate with a volume ratio of 2:1. After rotary evaporation at 40°C, it is vacuum-dried to obtain 2.21 g vanillin acetal compound, namely vanillin acetal; yield 78.1%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com