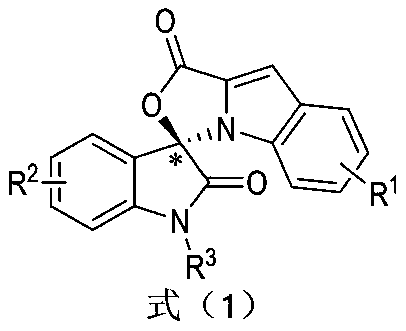
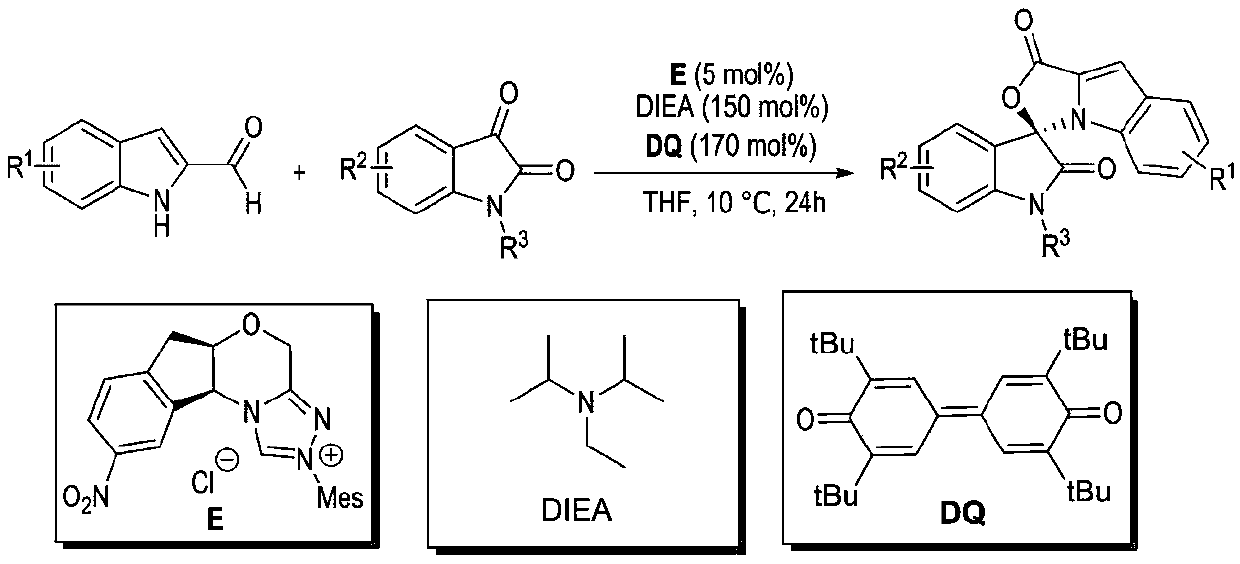
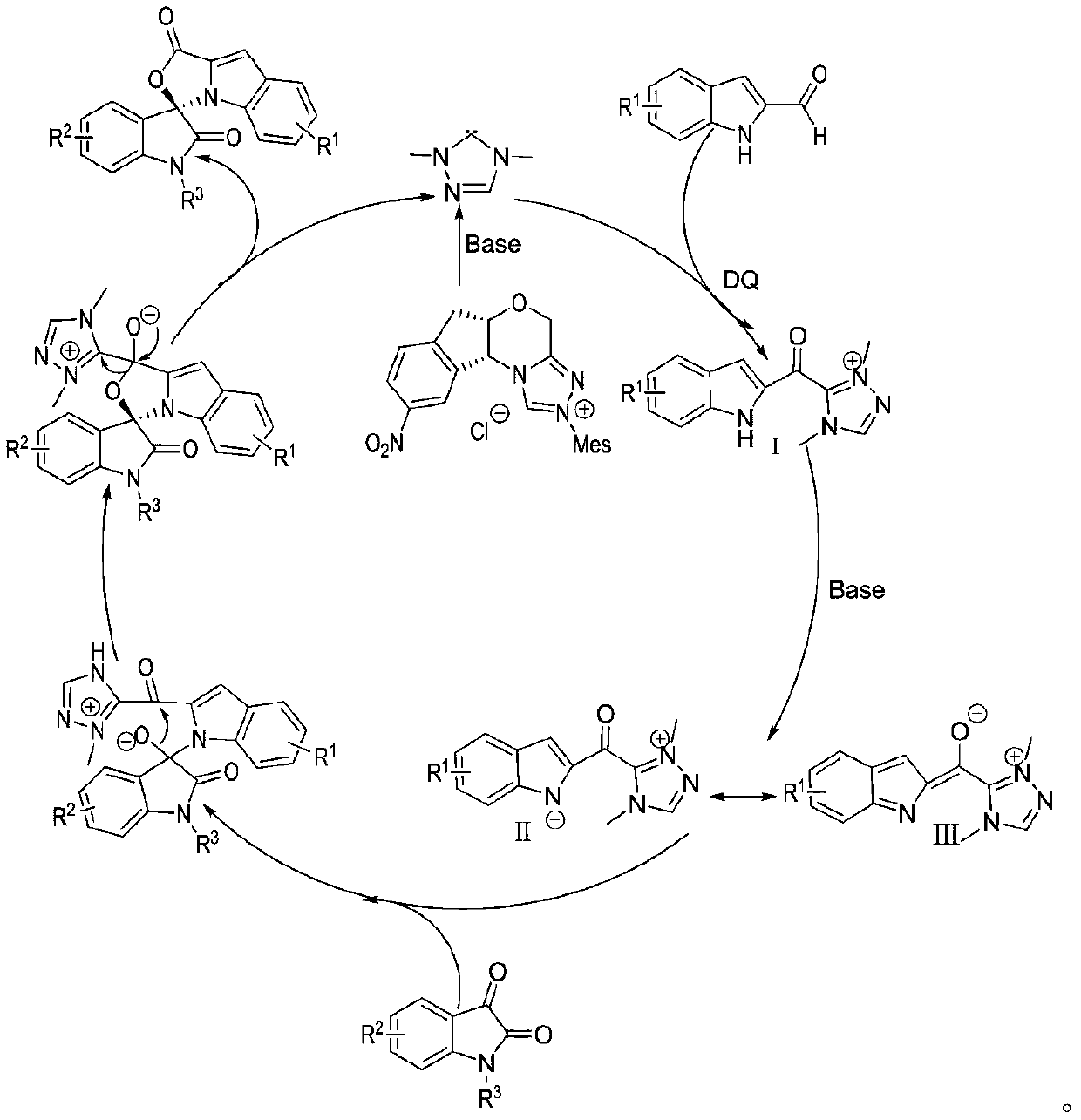
Preparation method and use of n-heterocyclic carbene catalyzed indole-containing skeleton chiral spiro compound
A technology for spiro compounds and indole, which is applied in the field of preparation and application of chiral spiro compounds containing indole skeleton catalyzed by nitrogen heterocyclic carbene, and achieves high enantioselectivity, excellent yield and good universality Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation Embodiment 1
[0031] Substituent R 1 for H,R 2 for H, R 3 For Bn, preparation implementation method and condition are with total embodiment 1;
[0032] Weigh respectively 0.15mmol (23.73mg) of indole-2-carboxaldehyde 1, 0.1mmol (21.86mg) of indole-2,3-dione 2, 0.05mmol (2.1mg) of nitrogen heterocyclic carbene catalyst E and 0.17mmol Add (69.5mg) oxidant DQ into a 10mL Schlenk reaction tube equipped with a magnetic stir bar, add 1mL solvent tetrahydrofuran THF, then add 0.15mmol (25μL) base N,N-diisopropylethylamine DIEA, gently shake the reaction wall , to mix thoroughly. Cover the bottle and place it in a 10°C isopropanol water bath to fully stir the reaction for 24h. After the completion of the reaction monitored by TLC, 1 mL of 1N hydrochloric acid was added to the reaction tube, stirred at room temperature for 5 minutes, the organic layer was extracted with ethyl acetate, spin-dried, a small amount of dichloromethane was fully dissolved and loaded by wet method, separated by column ...
preparation Embodiment 2
[0039] Substituent R 1 4-Br,R 2 for H, R 3 For Bn, preparation implementation method and condition are with total embodiment 1;
[0040] (R)-1-Benzyl-8'-bromo-1'H-spiro[indoline-3,3'-oxazolo[3,4-α]indole]-1',2dione (I 2 )
[0041] 7.07–6.92(m, 2H), 6.44(d, J=8.4Hz, 1H), 5.11(d, J=15.4Hz, 1H), 4.83(d, J=15.4Hz, 1H);
[0042] 13 C NMR (101MHz, CDCl 3 )δ166.9, 157.7, 142.7, 133.3, 132.8, 132.6, 131.1, 128.0, 127.3, 126.6, 125.6, 125.1, 124.3, 124.0, 123.3, 119.1, 116.7, 109.7, 108.2, 102.4, 87.1;
[0043] HRMS (ESI,m / z)calcd.for C 24 h 15 N 2 o 3 Br H + :459.0338,found:459.0332;
[0044] Chiral analysis was performed by HPLC, the specific conditions were: 97:3er (U-IC column, 25°C, hexans / i PrOH=90 / 10, 0.3mL / min, λ=254nm), Rt(minor)=17.3min, Rt(major)=14.3min.
preparation Embodiment 3
[0046] Substituent R 1 4-OCH 3 , R 2 for H, R 3 For Bn, preparation implementation method and condition are with total embodiment 1;
[0047] (R)-1-Benzyl-8'-methoxy-1'H-spiro[indoline-3,3'-oxazolo[3,4-α]indole]-1',2 Diketone (I 3 )
[0048] 1H), 5.11(d, J=15.5Hz, 1H), 4.82(d, J=15.5Hz, 1H), 3.95(s, 3H);
[0049] 13 C NMR (101MHz, CDCl 3 )δ167.9, 158.9, 155.2, 143.4, 134.1, 133.0, 128.7, 127.9, 127.3, 126.9, 125.7, 124.3, 123.9, 122.9, 120.3, 110.3, 102.5, 101.0, 100.8, 87.6, 53.1;
[0050] HRMS (ESI,m / z)calcd.for C 25 h 18 N 2 o 4 h + :411.1339,found:411.1329;
[0051] Chiral analysis was performed by HPLC, the specific conditions were: 97:3er (U-IC column, 25°C, hexans / i PrOH=90 / 10, 0.3mL / min, λ=254nm), Rt(minor)=41.9min, Rt(major)=32.9min.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com