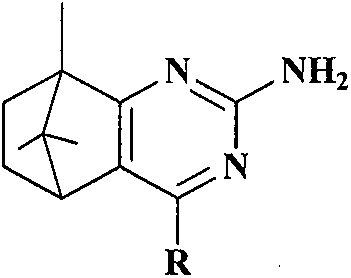
Synthesis and anti-tumor activity of camphoryl pyrimidines
A technology based on compound and pyrimidine, which is applied in the synthesis of camphor-based pyrimidine compounds and its anti-tumor activity, can solve the problems of high toxicity and side effects, achieve high selectivity and yield, high solvent recovery rate, and simple synthesis process Effect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
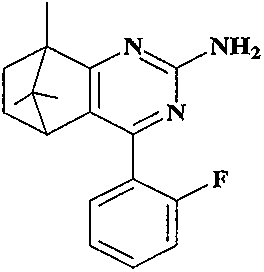
[0029] 4-(2'-fluorophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanoquinazolin-2-amine (compound 1 )
[0030]
[0031] In a 50ml three-necked flask equipped with a magnetic stirrer, a thermometer and a reflux condenser, add guanidine hydrochloride (8mmol), tert-butanol (35mL) and potassium tert-butoxide (12mmol) successively, and stir to make guanidine hydrochloride and potassium tert-butoxide Dissolve, add 3-(2-fluorobenzylidene) camphor (2 mmol), and heat to reflux for 10 h (TLC tracking detection). After the reaction, the reaction solution was concentrated to remove tert-butanol, added ethyl acetate, washed with distilled water and saturated brine to neutrality, and then washed with anhydrous Na 2 SO 4 Dry, filter and concentrate to obtain a brown crude product, which is recrystallized from methanol to obtain a white solid powder. Yield 92.6%; m.p.166.2~166.4℃; 1 H NMR (400MHz, CDCl 3 )δ: 7.57(s, 1H), 7.40(s, 1H), 7.13(s, 2H), 5.39(s, 2H), 2.79(s, 1H), 2.28(s, ...
Embodiment 2
[0033] 4-(3'-fluorophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanoquinazolin-2-amine (compound 2 )
[0034]
[0035] The preparation method is the same as in Example 1. Substitute 2-fluorobenzaldehyde with 3-fluorobenzaldehyde to obtain a white solid powder. Yield 88.9%; m.p.155.4~155.9℃; 1 H NMR (400MHz, CDCl 3 )δ: 7.49-7.55 (m, 2H), 7.39-7.44 (m, 1H), 7.09-7.14 (m, 1H), 5.31 (s, 2H), 3.06 (d, J=4Hz, 1H), 2.18- 2.23 (m, 1H), 1.86-1.96 (m, 1H), 1.32-1.37 (m, 2H), 1.25 (s, 3H), 0.99 (s, 3H), 0.63 (s, 3H); 13 C NMR (100MHz, CDCl 3 )δ: 182.24, 164.11, 161.75, 154.70, 140.38, 129.98, 125.48, 123.96, 116.18, 115.33, 55.96, 54.25, 49.65, 31.76, 25.95, 19.96, 18.98, 10.03; + ): m / z calculated for C 18 h 20 FN 3 [M+H] + 298.1720, found 298.1717.
Embodiment 3
[0037] 4-(4'-fluorophenyl)-8,9,9-trimethyl-5,6,7,8-tetrahydro-5,8-methanoquinazolin-2-amine (compound 3 )
[0038]
[0039] The preparation method is the same as in Example 1. Substitute 2-fluorobenzaldehyde with 4-fluorobenzaldehyde to obtain a white solid powder. Yield 91.5%; m.p.197.0~197.9℃; 1 H NMR (400MHz, CDCl 3)δ: 7.75-7.79(m, 2H), 7.14(t, J=8Hz, 2H), 5.27(s, 2H), 3.03(d, J=3.9Hz, 1H), 2.16-2.22(m, 1H) , 1.86-1.95(m, 1H), 1.32-1.36(m, 2H), 1.25(s, 3H), 0.99(s, 3H), 0.63(s, 3H); 13 C NMR (100MHz, CDCl 3 )δ: 181.98, 164.66, 161.74, 155.07, 134.17, 130.24, 125.17, 115.32, 55.97, 54.21, 49.70, 31.76, 25.97, 19.95, 19.01, 10.03; HR-MS (ESI + ): m / z calculated for C 18 h 20 FN 3 [M+H] + 298.1720, found 298.1717.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com