A kind of mangiferin cholic acid derivative and its preparation method and application
A technology of glycoside cholic acid and derivatives, which is applied in the field of mangiferin cholic acid derivatives and its preparation, can solve the problem that esterified derivatives cannot be used as targeted preparations for the treatment of liver and gallbladder inflammation, and achieve the purpose of improving the inhibition of liver and gallbladder inflammation and improving the efficacy of drugs. effect of concentration
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
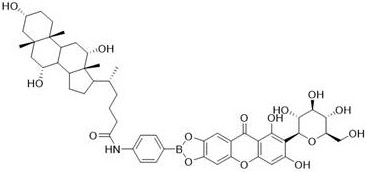
[0035] Described mangiferin cholic acid derivative, its preparation method comprises the following steps:
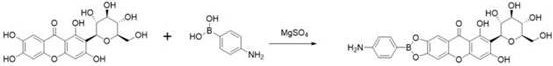
[0036] (1) Mangiferin and p-aminophenylboronic acid were fed at a ratio of 1:1, anhydrous magnesium sulfate was used as a catalyst, and reacted in an anhydrous organic solvent, stirred at room temperature to react, and the reaction process was detected by thin-layer chromatography; the reaction After completion, filter, freeze-dry, and recrystallize with DMSO to obtain the primary product;
[0037] (2) Take the primary product obtained in step (1), mix it with 1.2 equivalents of cholic acid, use EDCI as a dehydrating agent, use DMAP as a catalyst, react in an anhydrous organic solvent, stir evenly at room temperature, and monitor with a thin-layer chromatography plate Reaction process; after the reaction, add organic solvent dichloromethane or chloroform, wash with saturated brine for several times, and dry in vacuum to obtain crude product;
[0038] (3) Take the crude ...
Embodiment 2
[0042] Described mangiferin cholic acid derivative, its preparation method comprises the following steps:
[0043] (1) Take 1g of mangiferin, 324mg of p-aminophenylboronic acid, and 5g of anhydrous magnesium sulfate, react in 30mL of anhydrous organic solvent, stir at room temperature to react, use thin-layer chromatography to detect the reaction process; filter after the reaction, Freeze-dried and recrystallized with DMSO to obtain the primary product;
[0044](2) Take the primary product obtained in step (1), mix it with 1.2 equivalents of cholic acid, use EDCI as a dehydrating agent, use DMAP as a catalyst, react in an anhydrous organic solvent, stir evenly at room temperature, and monitor with a thin-layer chromatography plate Reaction process; after the reaction, add organic solvent dichloromethane or chloroform, wash with saturated brine for several times, and dry in vacuum to obtain crude product;
[0045] (3) Take the crude product obtained in step (2), and recrystall...
Embodiment 3
[0049] Described mangiferin cholic acid derivative, its preparation method comprises the following steps:
[0050] (1) Take 1g of mangiferin, 324mg of p-aminophenylboronic acid, and 5g of anhydrous magnesium sulfate, react in 30mL of anhydrous organic solvent, stir at room temperature to react, use thin-layer chromatography to detect the reaction process; filter after the reaction, Freeze-dried and recrystallized with DMSO to obtain the primary product;
[0051] (2) Take the primary product obtained in step (1), mix it with 1.2 equivalents of cholic acid, use EDCI as a dehydrating agent, use DMAP as a catalyst, react in an anhydrous organic solvent, stir evenly at room temperature, and monitor with a thin-layer chromatography plate Reaction process; after the reaction, add organic solvent dichloromethane or chloroform, wash with saturated brine for several times, and dry in vacuum to obtain crude product;
[0052] (3) Take the crude product obtained in step (2), and recrystal...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com