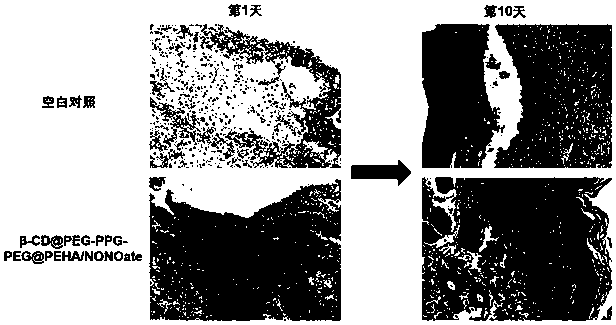
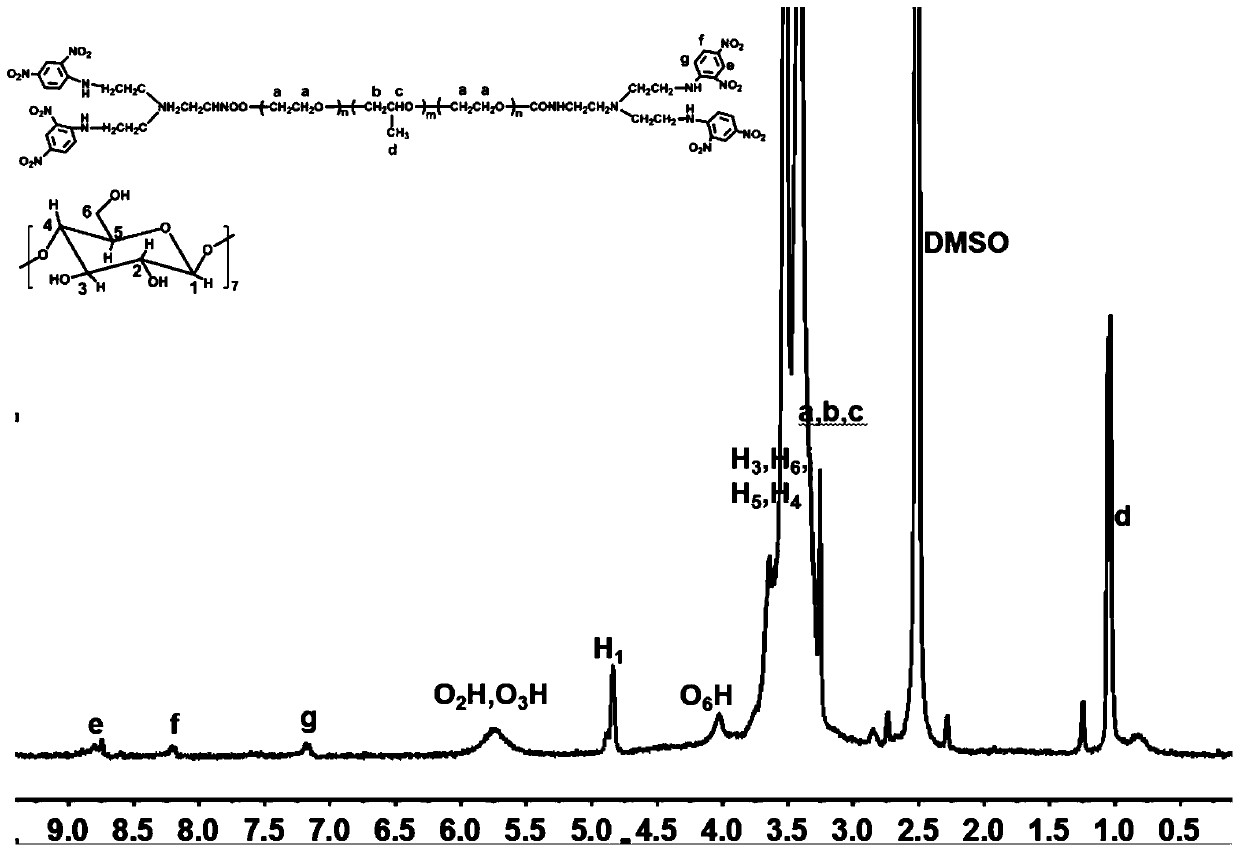
Polyrotaxane-structured NO donor material and preparation method and application thereof
A polyrotaxane, donor technology, applied in the field of biomedical engineering materials, can solve problems such as neglect
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0078] (1) The anhydrous triblock copolymer PEG-PPG-PEG was added to anhydrous dichloromethane under nitrogen protection and dissolved completely. Then, p-toluenesulfonyl chloride was added at 5°C, and the stirring was continued for 20 minutes, followed by the addition of 4-dimethylaminopyridine (DMAP) and triethylamine (NeT 3 ), the reaction was continued for 48 h, the reaction was terminated, and 30 mL of saturated NaHCO was used. 3 Washed twice, evaporated to remove dichloromethane, and vacuum-dried at 50 °C for 24 h to obtain p-toluenesulfonylated PEG-PPG-PEG(PEG-(OTs) 2 );
[0079] Among them, the number average molecular weight of the anhydrous triblock copolymer PEG-PPG-PEG is 1000, and m:n is 1:0.5; the anhydrous triblock copolymer PEG-PPG-PEG, p-toluenesulfonyl chloride, 4-dimethylformaldehyde Aminopyridine (DMAP) and triethylamine (NeT 3 ) in a molar ratio of 1:2:0.05:5; the anhydrous dichloromethane consumption is calculated by dissolving 0.5g of anhydrous triblo...
Embodiment 2
[0092] PEG-PPG-PEG amination (PEG-PPG-PEG-NH 2 )preparation
[0093] (1) Add the anhydrous triblock copolymer PEG-PPG-PEG to anhydrous dichloromethane under nitrogen protection and dissolve it completely; then add p-toluenesulfonyl chloride at 20°C and continue to stir to stabilize After 30min, continue to add 4-dimethylaminopyridine (DMAP) and triethylamine (NeT 3 ), the reaction was continued for 72 h, the reaction was terminated, and 60 mL of saturated NaHCO was used. 3 Washed 3 times, evaporated to remove dichloromethane, and then vacuum-dried at 60 °C for 36 h to obtain p-toluenesulfonated PEG-PPG-PEG(PEG-(OTs) 2 );
[0094] Among them, the number average molecular weight of the anhydrous triblock copolymer PEG-PPG-PEG is 15000, and m:n is 1:1.5; the anhydrous triblock copolymer PEG-PPG-PEG, p-toluenesulfonyl chloride, 4-dimethylformaldehyde Aminopyridine (DMAP) and triethylamine (NeT 3 ) molar ratio is 1:10:0.1:15; the amount of dichloromethane is calculated by diss...
Embodiment 3
[0111] Take 1 mg of triblock copolymer PEG-PPG-PEG (raw material in Example 1) and the aminated PEG-PPG-PEG (PEG-PPG-PEG-NH) prepared in Example 1 respectively. 2 ), interlocking supramolecular polyrotaxane (β-CD@PEG-PPG-PEG-DNFB), cationized interlocking supramolecular polyrotaxane (β-CD@PEG-PPG-PEG@PEHA) and interlocking Supramolecular polyrotaxane NO donor (β-CD@PEG-PPG-PEG@PEHA / NONOate) was dispersed in 1 mL of pure water, and the surface potential changes of the corresponding materials were measured. The results are as follows: image 3 As shown, it was found that the potential of the aminated PEG-PPG-PEG changed significantly, proving that tris(2-aminoethyl)amine was successfully modified to both ends of PEG-PPG-PEG. The interlocked supramolecular polyrotaxane (β- The potential of CD@PEG-PPG-PEG-DNFB) was compared with that of aminated PEG-PPG-PEG (PEG-PPG-PEG-NH 2 ) was significantly reduced, and was significantly lower than that of PEG-PPG-PEG, which proved that the ...
PUM
| Property | Measurement | Unit |
|---|---|---|
| concentration | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com