Crystal form of pyrazoloheteroaryl derivative and preparation method of crystal form of pyrazoloheteroaryl derivative
A crystal form and crystallization technology, applied in the direction of organic chemical methods, medical preparations containing active ingredients, drug combinations, etc., can solve the problems of poor fluidity, fine crystallization, difficult filtration, etc.
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0119] Embodiment 1, the preparation of A crystal form
[0120] Crude compound 1f (37.9 g, 99.6 mmol) was added to 379 mL of trifluoroacetic acid, protected by argon replacement, 8.6 mL of concentrated sulfuric acid was added dropwise, heated to 80° C., and stirred until the reaction was complete. The reaction solution was concentrated under reduced pressure, 700 mL of dichloromethane was added to the resulting residue, and saturated sodium bicarbonate solution was added dropwise until the pH of the reaction solution was 7-8. Wash once each with saturated sodium bicarbonate solution and 500 mL saturated saline, dry with anhydrous sodium sulfate, filter to remove the desiccant, concentrate the filtrate under reduced pressure, and purify the obtained product by silica gel column chromatography with eluent system (dichloromethane / methanol) As a residue, the title product 1 was obtained (10 g, yield: 26.4%).
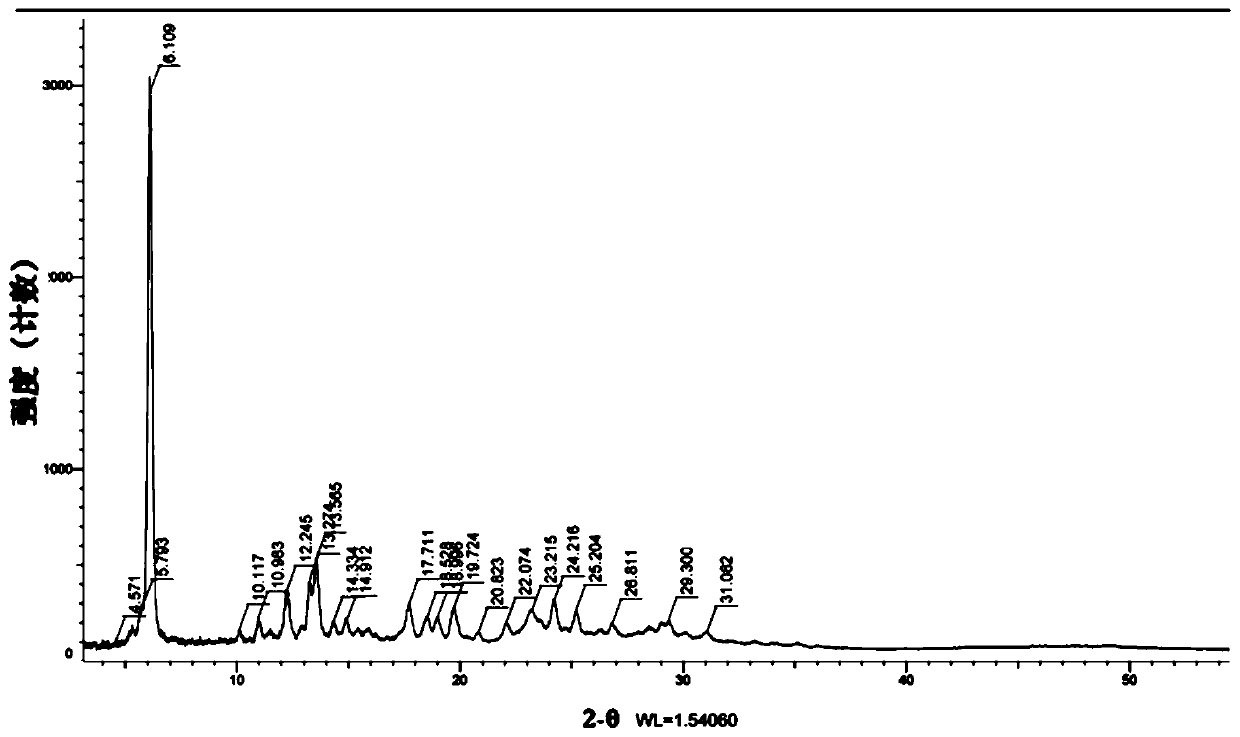
[0121] Detected by X-ray powder diffraction, the product is defined as...
Embodiment 2
[0125] Embodiment 2, the preparation of B crystal form
[0126] The compound represented by formula (I) (500 mg, 1.31 mmol) was dissolved in 5 mL of acetonitrile, heated to 80° C. and stirred for 30 minutes, filtered to remove insoluble matter, and the filtrate was naturally lowered to room temperature and stirred for 72 hours. The reaction solution was filtered, and the filter cake was collected and dried in vacuo to obtain the title product (400 mg, yield: 80%).
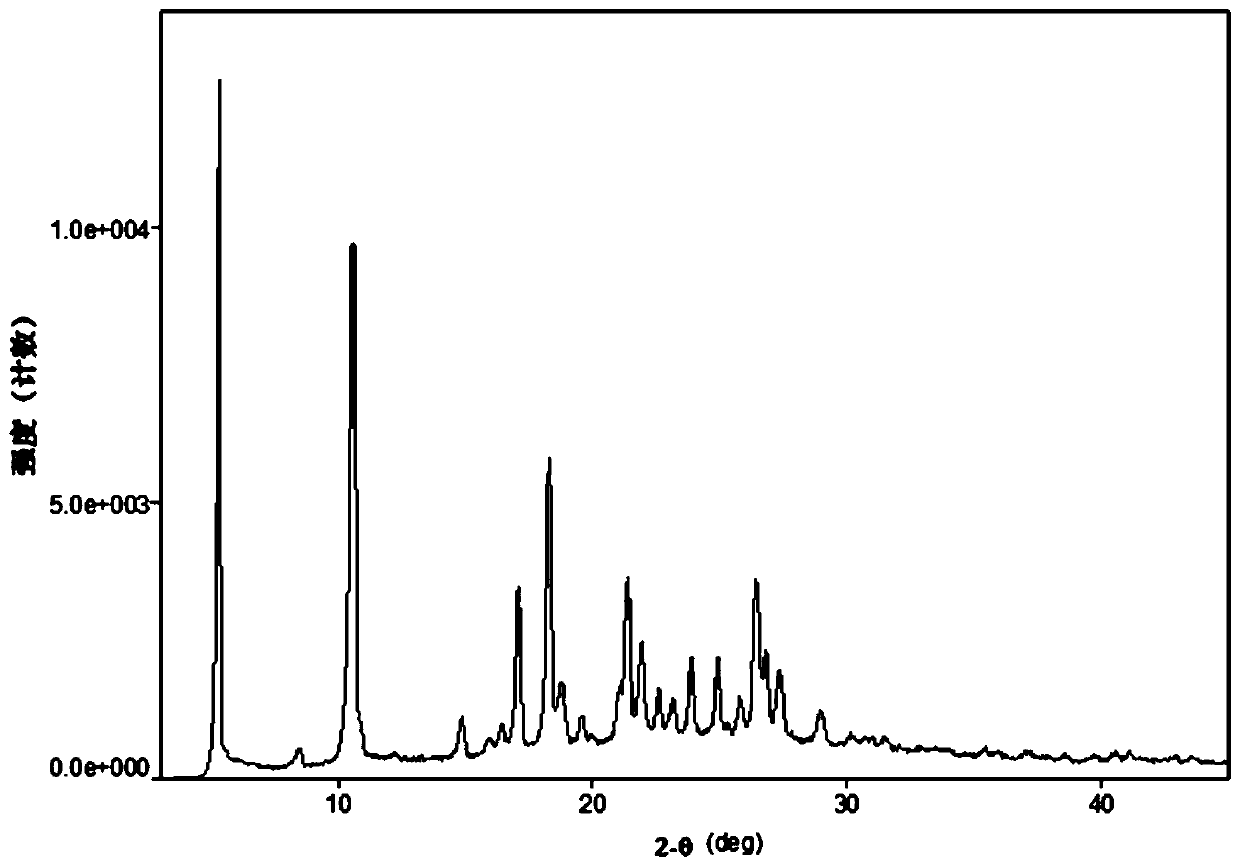
[0127] Through X-ray powder diffraction detection, the product is defined as crystal form B, and the XRPD spectrum is as follows: image 3 shown.
[0128] Table 2. Characteristic peaks of crystal form B
[0129]
[0130]
[0131] DSC spectrum such as Figure 4 As shown, it shows that the melting endothermic peak begins to appear at about 114.14°C, and the peak value is 115.83°C.
[0132] TGA spectrum such as Figure 5 As shown, it is anhydrous ansolvate.
[0133] DVS spectrum such as Figure 6 As shown...
Embodiment 3
[0134] Embodiment 3, the preparation of B crystal form
[0135] The compound represented by formula (I) (304 mg, 0.80 mmol) was dissolved in 3 mL of isopropyl acetate, heated to 80° C. and stirred for 30 minutes, filtered to remove insoluble matter, and the filtrate was naturally lowered to room temperature and stirred for 16 hours. The reaction solution was filtered, the filter cake was collected, and dried in vacuo to obtain the title product (200 mg, yield: 66%), which was found to be crystal form B by X-powder diffraction detection.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com