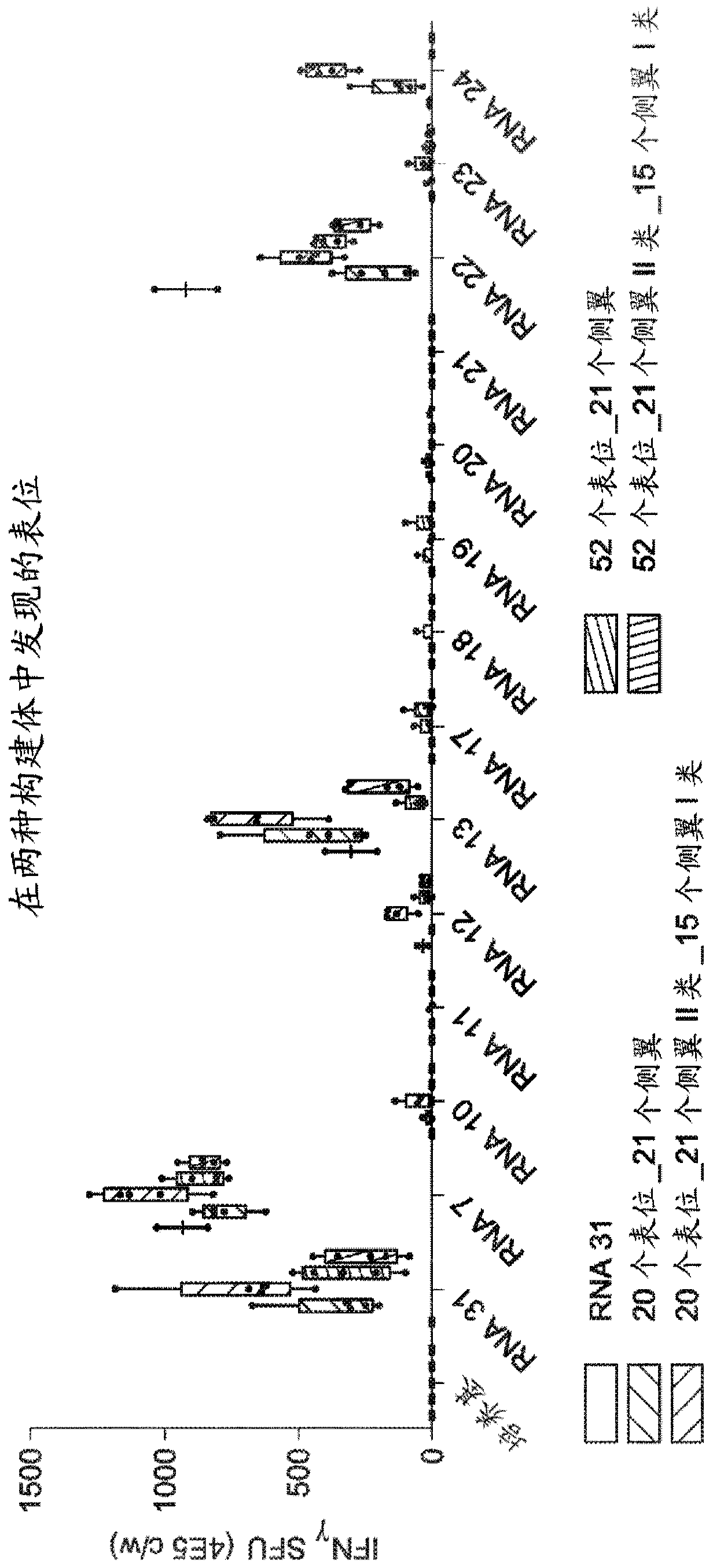
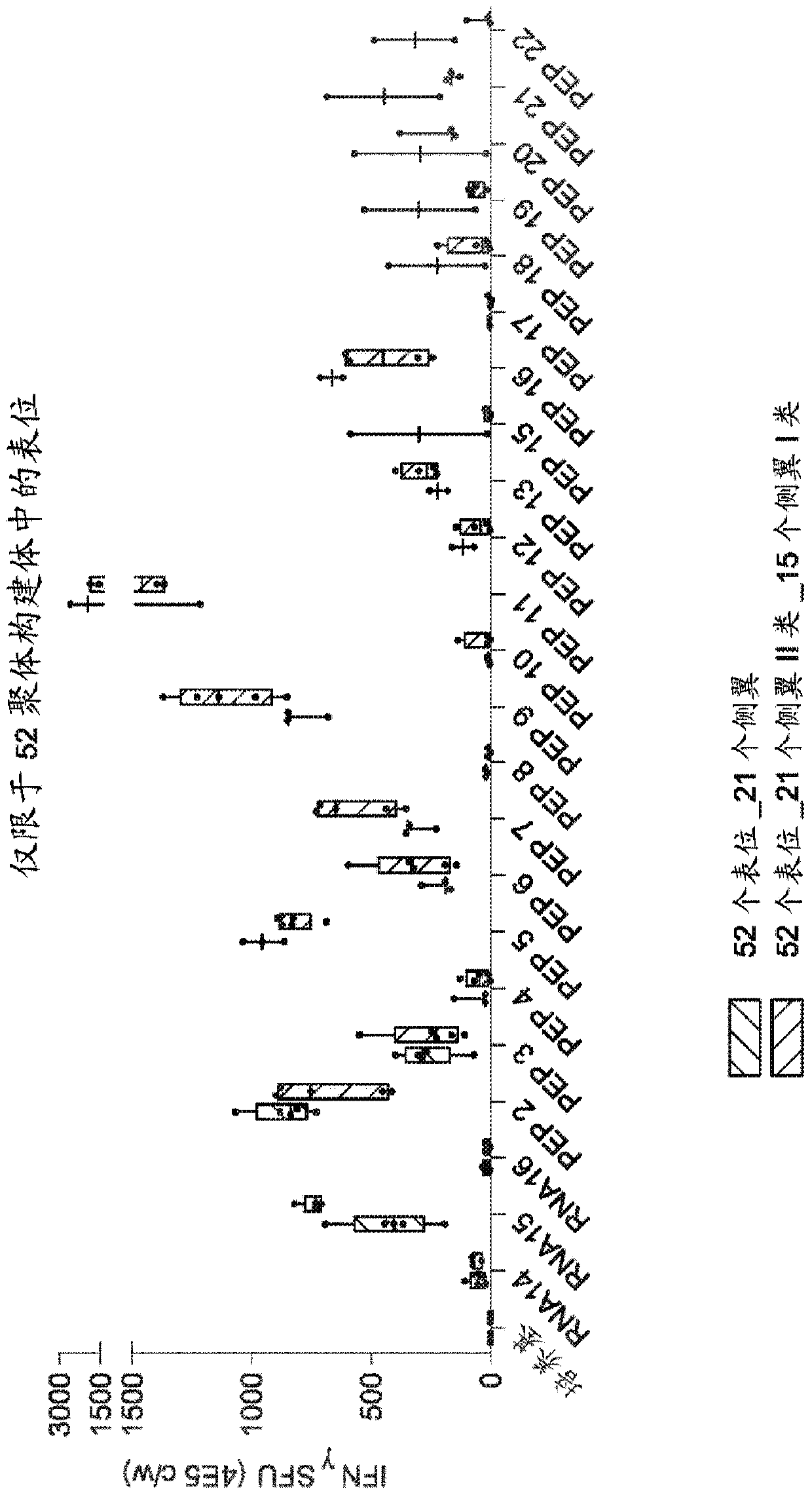
RNA cancer vaccines
A cancer vaccine and vaccine technology, applied in the field of RNA cancer vaccines, can solve problems such as inhibition, activation, and insertional mutagenesis
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
preparation example Construction
[0678] Formulations of the vaccine compositions described herein can be prepared by any method known or later developed in the art of pharmacology. In general, such methods of preparation comprise the steps of bringing into association the active ingredient (e.g., mRNA polynucleotide) with excipients and / or one or more other auxiliary ingredients, followed by, if necessary and / or desirable, Divide, shape and / or package the product into desired single or multi-dose units.
[0679] Cancer RNA vaccines can be formulated using one or more excipients to: (1) increase stability; (2) increase cell transfection; (3) allow sustained or delayed release (e.g., from a depot formulation); (4) Alter biodistribution (eg, targeting specific tissues or cell types); (5) Increase in vivo translation of the encoded protein; and / or (6) Alter the in vivo release profile of the encoded protein (antigen). In addition to conventional excipients such as any and all solvents, dispersion media, diluents...
Embodiment 1
[1567] Example 1. Production of polynucleotides
[1568] According to the present disclosure, the manufacture of polynucleotides and parts or regions thereof can be achieved using the methods taught in the International Application WO2014 / 152027 entitled "Manufacturing Methods for Production of RNA Transcripts", the contents of which are incorporated by reference Incorporated into this article as a whole.
[1569] Purification methods may include those taught in International Applications WO2014 / 152030 and WO2014 / 152031, each of which is incorporated herein by reference in its entirety.
[1570] Methods of detection and characterization of polynucleotides can be performed as taught in WO2014 / 144039, which is hereby incorporated by reference in its entirety.
[1571] Characterization of polynucleotides of the present disclosure can be accomplished using a program selected from the group consisting of polynucleotide mapping, reverse transcriptase sequencing, charge distributi...
Embodiment 2
[1572] Embodiment 2 chimeric polynucleotide synthesis
[1573] introduction
[1574] According to the present disclosure, two regions or portions of a chimeric polynucleotide can be joined or linked using triphosphate chemistry.
[1575] According to this method, a first region or portion of 100 nucleotides or less is chemically synthesized with a 5' monophosphate and a terminal 3' desOH or blocked OH. If the region is longer than 80 nucleotides, it can be synthesized as two strands for ligation.
[1576] If the first region or portion is synthesized using in vitro transcription (IVT) as a non-position-modified region or portion, then conversion to the 5' monophosphate and subsequent capping of the 3' end may follow.
[1577] The monophosphate protecting group can be selected from any one known in the art.
[1578] The second region or portion of the chimeric polynucleotide can be synthesized using chemical synthesis or IVT methods. IVT methods can include RNA polymerase...
PUM
| Property | Measurement | Unit |
|---|---|---|
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
| diameter | aaaaa | aaaaa |
Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com