Method for preparing 2, 2-dimethyl-1, 3-epoxypropane
A technology of propylene oxide and dimethyl group, which is applied in the field of preparation of organic chemical intermediates, can solve the problems of difficult solid-liquid separation operation, insufficient saponification reaction, and high impurity boiling point, achieves good mass transfer and heat transfer effect, and suppresses side effects. Simple effect of product generation and solid-liquid separation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0038] (1) Refining and purifying of the by-product 2,2-dimethyl-3-chloropropanol produced by ibuprofen:
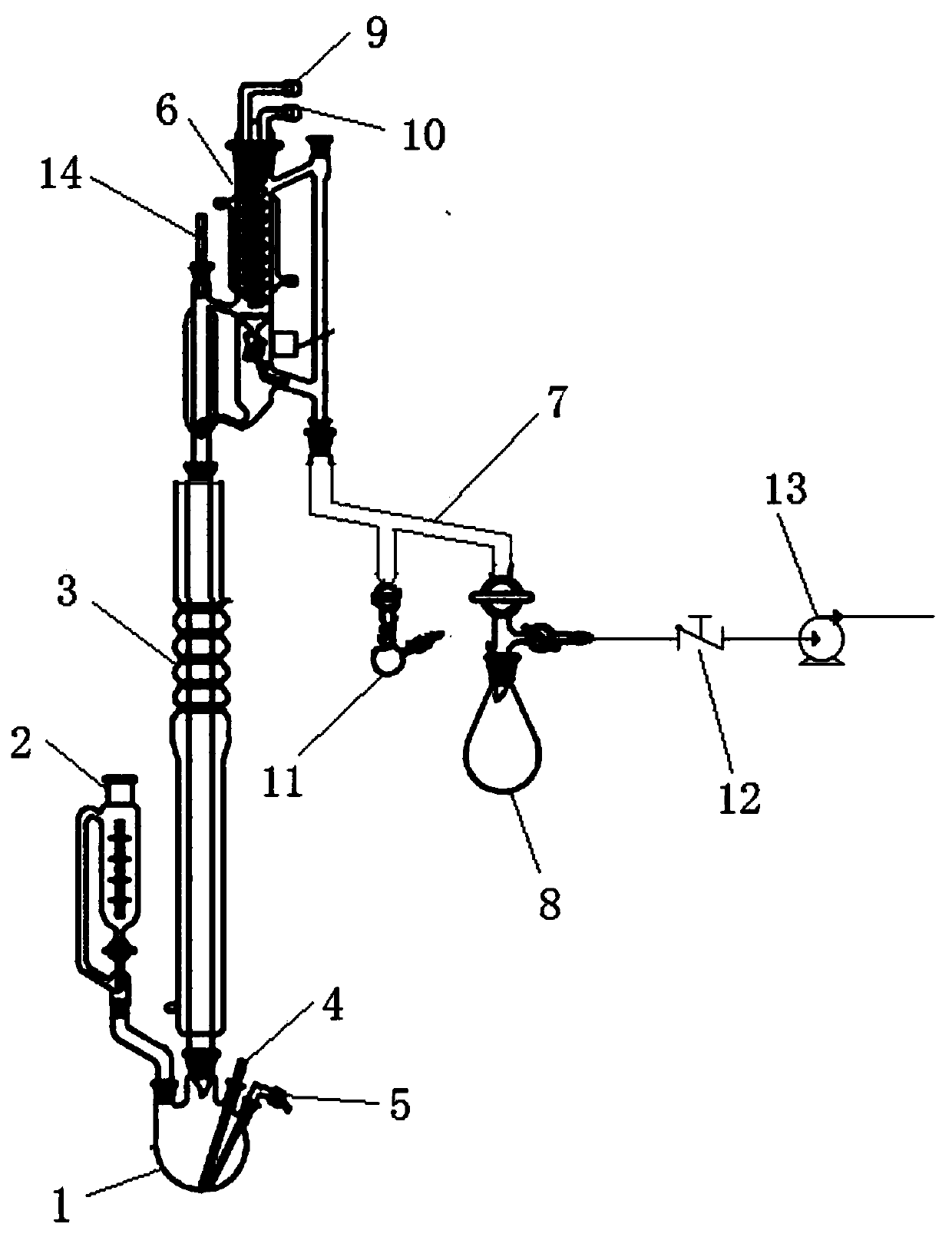
[0039] use figure 1For the shown rectification device, use a vacuum pump to evacuate, control the absolute pressure in the four-necked flask one at 20kPa, put cooling water into the condenser one, heat the four-necked flask one, and keep the rectifying temperature at 150°C. After 1 hour, the temperature was raised to 180°C for 0.5 hours, and 2,2-dimethyl-3-chloropropanol was evaporated from the top of the device into the receiving bottle 1 with a purity of 95%; when the temperature at the top of the device reached 130°C , turn off the vacuum pump, open the vent valve, balance the internal and external pressure of the system, and put the residual liquid into the waste liquid bucket.
[0040] (2) Saponification reaction of 2,2-dimethyl-3-chloropropanol:
[0041] The mass concentration is 35% sodium hydroxide solution and phase transfer catalyst dodecyltrimethylammonium br...
Embodiment 2
[0045] (1) Refining and purifying of the by-product 2,2-dimethyl-3-chloropropanol produced by ibuprofen:
[0046] use figure 1 For the shown rectification device, use a vacuum pump to evacuate, control the absolute pressure in the four-necked flask one at 10kPa, feed cooling water into the condenser one, heat the four-necked flask one, and keep the rectification temperature at 13°C. After 2 hours, the temperature was raised to 160°C for 1 hour, and 2,2-dimethyl-3-chloropropanol was evaporated from the top of the device into the receiving bottle 1 with a purity of 96%; when the temperature at the top of the device reached 120°C , turn off the vacuum pump, open the vent valve, balance the internal and external pressure of the system, and put the residual liquid into the waste liquid bucket.
[0047] (2) Saponification reaction of 2,2-dimethyl-3-chloropropanol:
[0048] Get the waste lye 680g after the filtration that embodiment one obtains, record its sodium hydroxide content ...
Embodiment 3
[0053] (1) Refining and purifying of the by-product 2,2-dimethyl-3-chloropropanol produced by ibuprofen:
[0054] use figure 1 For the shown rectification device, use a vacuum pump to evacuate, control the absolute pressure in the four-necked flask one at 30kPa, put cooling water into the condenser one, heat the four-necked flask one, and keep the rectification temperature at 120°C. After 2.5 hours, the temperature was raised to 170°C for 1 hour, and 2,2-dimethyl-3-chloropropanol was evaporated from the top of the device into the receiving bottle 1 with a purity of 95.6%; when the temperature at the top of the device reached 125°C , turn off the vacuum pump, open the vent valve, balance the internal and external pressure of the system, and put the residual liquid into the waste liquid bucket.
[0055] (2) Saponification reaction of 2,2-dimethyl-3-chloropropanol:
[0056] Get the filtered waste lye 600g that embodiment one obtains, record its sodium hydroxide content 37.5%, a...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D Engineer
- R&D Manager
- IP Professional
- Industry Leading Data Capabilities
- Powerful AI technology
- Patent DNA Extraction
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2024 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com