Method for separating neogambogic acid and gambogic acid by medium and low pressure gradient silica gel dry column chromatography and method for preparing N-aryl garcinamide
A technology for separation of aryl gambogic amides and column chromatography, which is applied in organic chemistry and other fields, can solve the problems of limited separation technology popularization and high equipment cost, and achieve the effects of reducing three wastes, simple method and ensuring stability
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
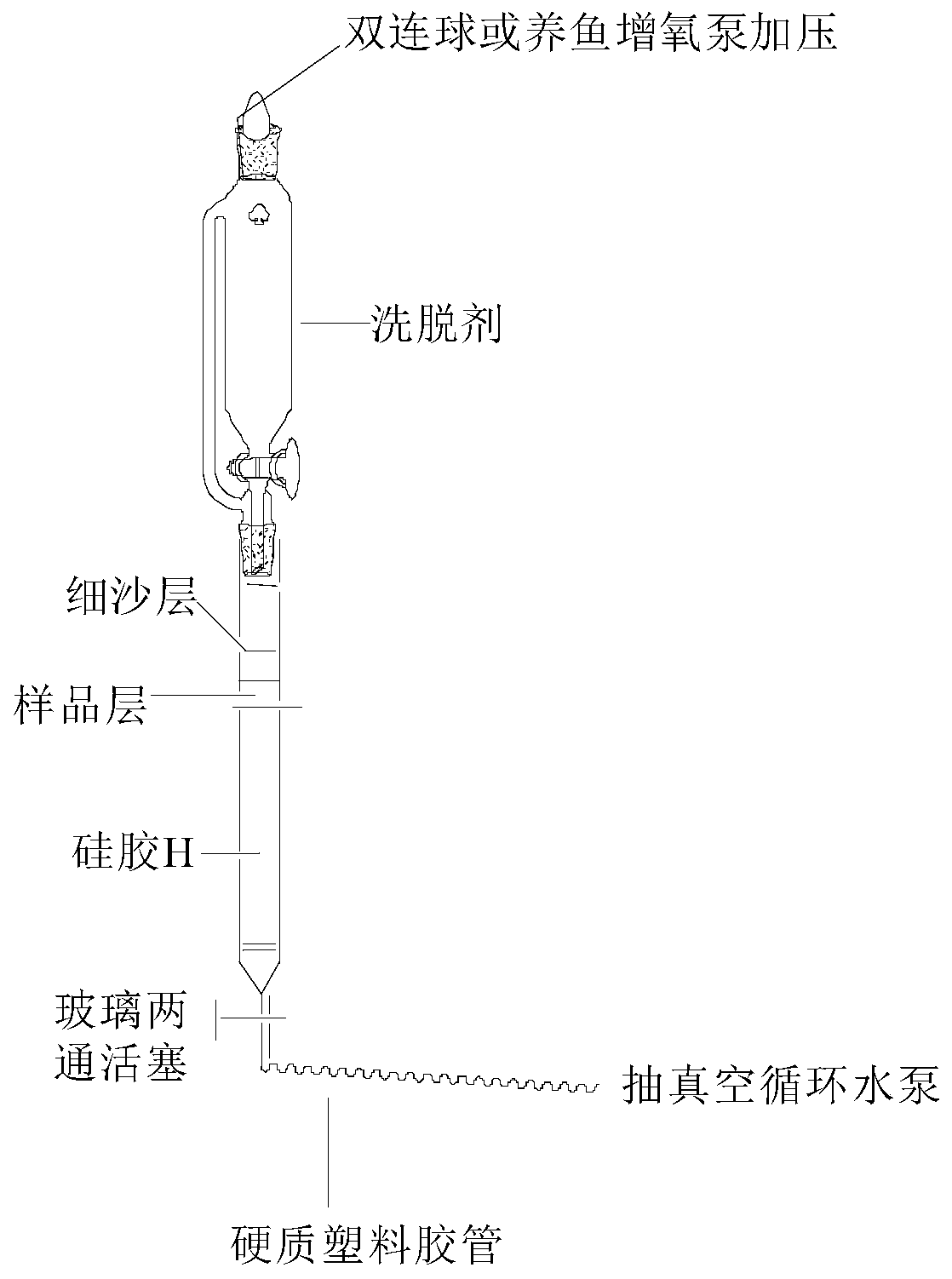
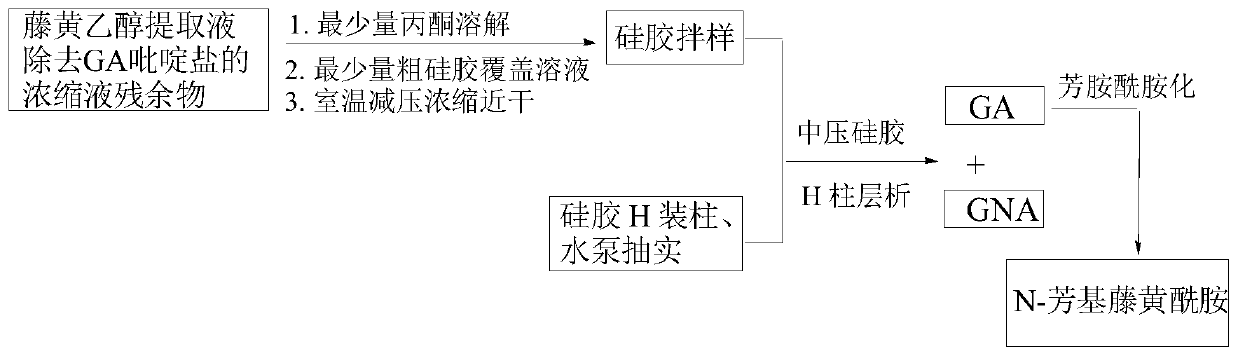
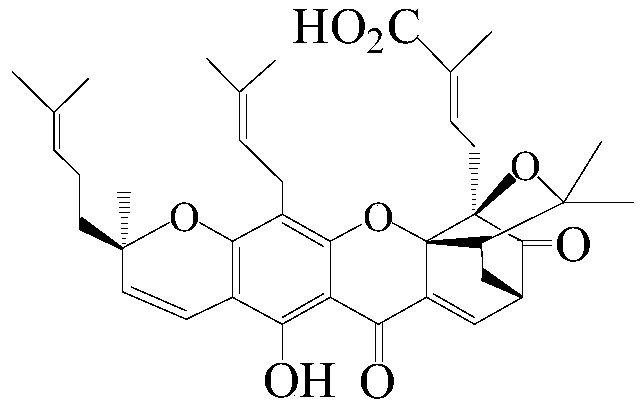
[0049] The present invention passes figure 1 The separation device shown (the chromatographic column is equipped with silica gel H) selects dichloroethane-ethanol-triethylamine system as the eluent for one-time medium and low pressure gradient silica gel H dry column chromatography, which can be filtered out from the garcinia extract High-purity GA and GNA were isolated from the mother liquor of gambogic acid pyridinium salt.
[0050] The specific process steps are as follows:
[0051] (1) in such as figure 1 The chromatographic column with a piston at the bottom and a sand-permeable plate at the upper end is sequentially filled with silica gel H activated at 110°C for 2 hours [silica gel height: inner diameter>8:1, and the weight ratio of the sample to be separated (60-90): 1] After mixing the sample with coarse silica gel and 2-3mm fine sand layer, install a constant pressure dropping funnel with a piston and eluent, and a water pump for pumping;
[0052] (2) Shut down th...
Embodiment 2
[0060] EDCI.HCl, DMAP jointly catalyze the method for preparing N-aryl gambogic amide by reacting gambogic acid and arylamine, comprising the following steps:
[0061] (1) GA (initial concentration of 4.9mmol / L), 1-ethyl-3-(N,N-dimethylamino) propylcarbodiimide hydrochloride (EDCI.HCl, 1.1equiv., 5.5mmol / L initial concentration), 4-(N,N-dimethyl)pyridine (DMAP, 0.2equiv.,, 0.9mmol / L initial concentration) and dichloromethane (DCM) stirred at room temperature for 15min, then added 4- Chloro-3-trifluoromethylaniline (1.1 equiv., initial concentration of 4.8 mmol / L) in dichloromethane. Stirring was continued at room temperature for 11 h until the gambogic acid spots in the reaction solution were detected by silica gel TLC (developing agent: dichloromethane-ethanol-triethylamine v / v 80:0.1:3) completely disappeared;
[0062] (2) After adding water to quench the reaction, the organic layer was separated, washed with water (4 mL×2), and the organic phase was separated and dried ov...
Embodiment 3
[0064] EDCI.HCl, DMAP jointly catalyze the method for preparing N-aryl gambogic amide by reacting gambogic acid and arylamine, comprising the following steps:
[0065] (1) GA (initial concentration of 4.9mmol / L), 1-ethyl-3-(N,N-dimethylamino) propylcarbodiimide hydrochloride (EDCI.HCl, 1.1equiv., 5.5mmol / L initial concentration), 4-(N,N-dimethyl)pyridine (DMAP, 0.2equiv., 0.9mmol / L initial concentration) and dichloromethane (DCM) stirred at room temperature for 15min, then added 3-( 6-Chloropyridyl)amine (1.1 equiv., initial concentration of 4.8 mmol / L) in dichloromethane. Stirring was continued at room temperature for 12 hours until the GA spots in the reaction solution were detected by silica gel TLC (developing solvent: dichloromethane-ethanol-triethylamine v / v 80:0.1:3) completely disappeared;
[0066] (2) After adding water to quench the reaction, the organic layer was separated, washed with water (4 mL×2), and the organic phase was separated and dried over anhydrous ma...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com