Preparation method of tadalafil and intermediate of tadalafil
A tadalafil and intermediate technology, applied in the field of chemical pharmacy, can solve the problems of increased cost, complicated preparation process, inability to convert, etc., and achieves the effects of reducing solvent loss, improving reaction rate, and facilitating operation
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
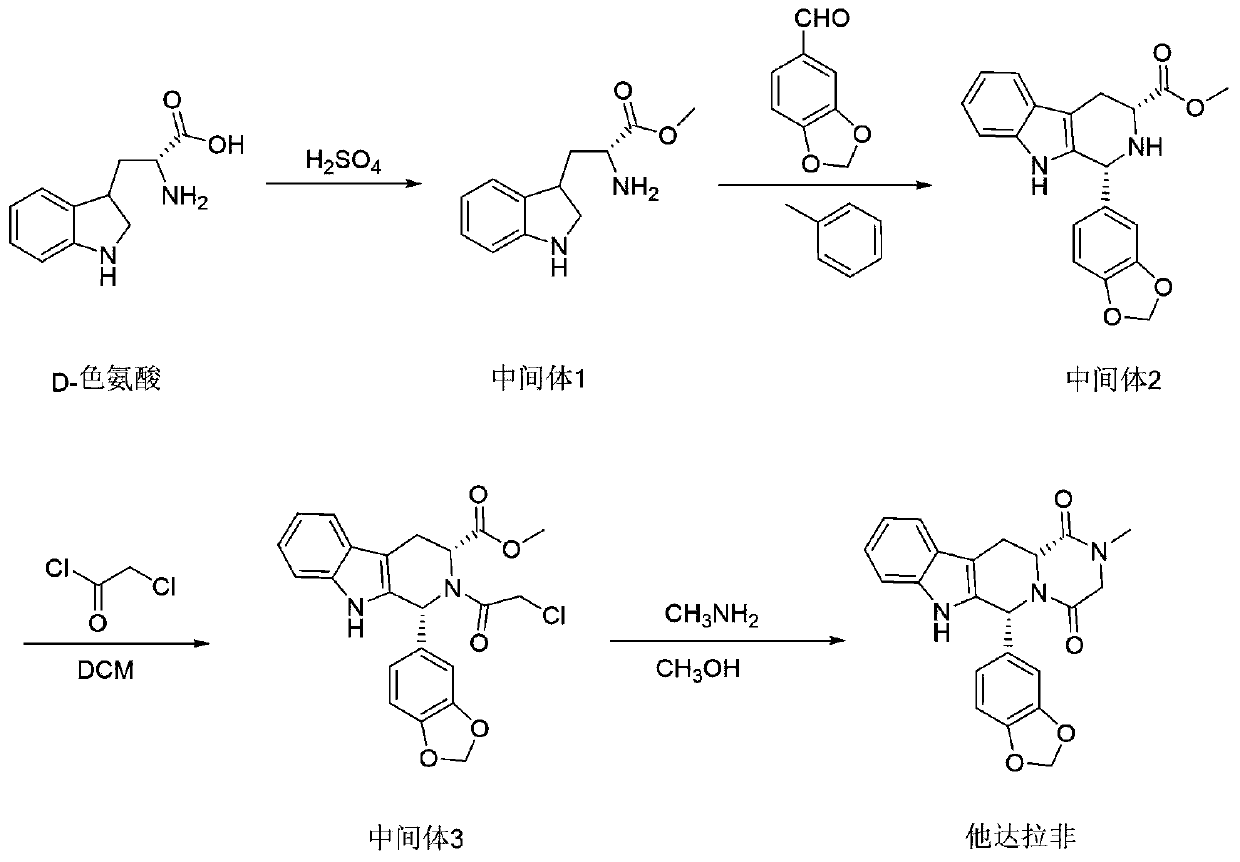
[0027] Synthesis of Intermediate 1
[0028] Add D-tryptophan (25kg, 123mol) and methanol 125L into a 500L reaction kettle, stir at room temperature, slowly add sulfuric acid (588g, 6mol) dropwise, after the dropwise completion, heat to reflux, keep the reaction for 2h, HPLC detection is completed, evaporate under reduced pressure In addition to methanol, 175 L of ethyl acetate was added to beat for 2 hours, centrifuged, and the product was dried by double cones to obtain 31 kg of off-white solid with a yield of 99.4% and a purity of 99.1%.
Embodiment 2
[0030] Synthesis of Intermediate 1
[0031] Add D-tryptophan (25kg, 123mol) and methanol 125L into a 500L reactor, stir at room temperature, slowly add sulfuric acid (362g, 3.7mol) dropwise, after the dropwise completion, heat to reflux, keep warm for 3h, after HPLC detection is completed, depressurize Distill methanol off, add 175 L of ethyl acetate to beat for 2 hours, centrifuge, and dry the product to obtain 30.8 kg of intermediate type 1 white solid with a yield of 98.9% and a purity of 99%.
Embodiment 3
[0033] Synthesis of Intermediate 1
[0034] Add D-tryptophan (25kg, 123mol) and methanol 125L into a 500L reaction kettle, stir at room temperature, slowly add sulfuric acid (960g, 9.8mol) dropwise, after the dropwise completion, heat to reflux, keep the reaction for 2h, HPLC detection is completed, depressurize Evaporate methanol, add 175 L of ethyl acetate to beat for 2 hours, centrifuge, and dry the product to obtain 30.9 kg of intermediate type 1 white solid, with a yield of 99.2% and a purity of 98.9%.
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - Generate Ideas
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com