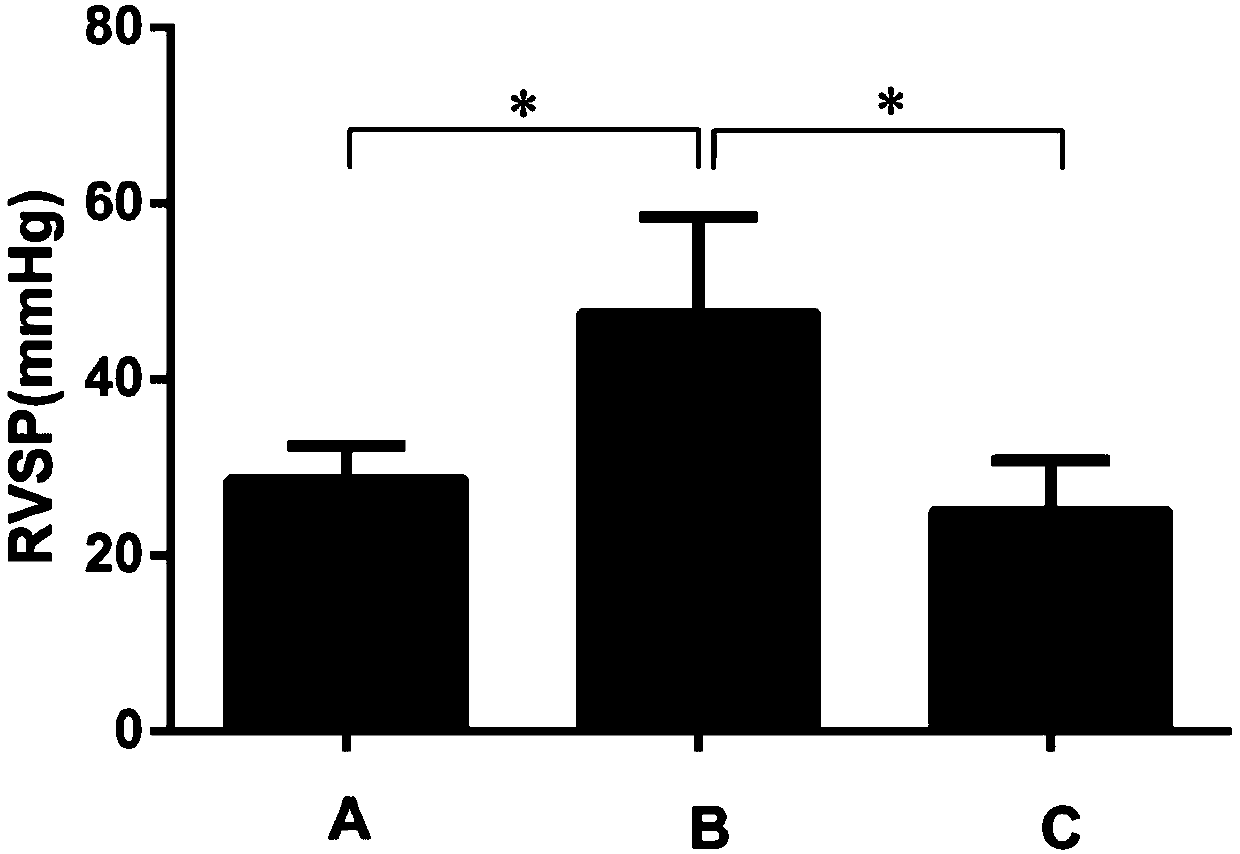
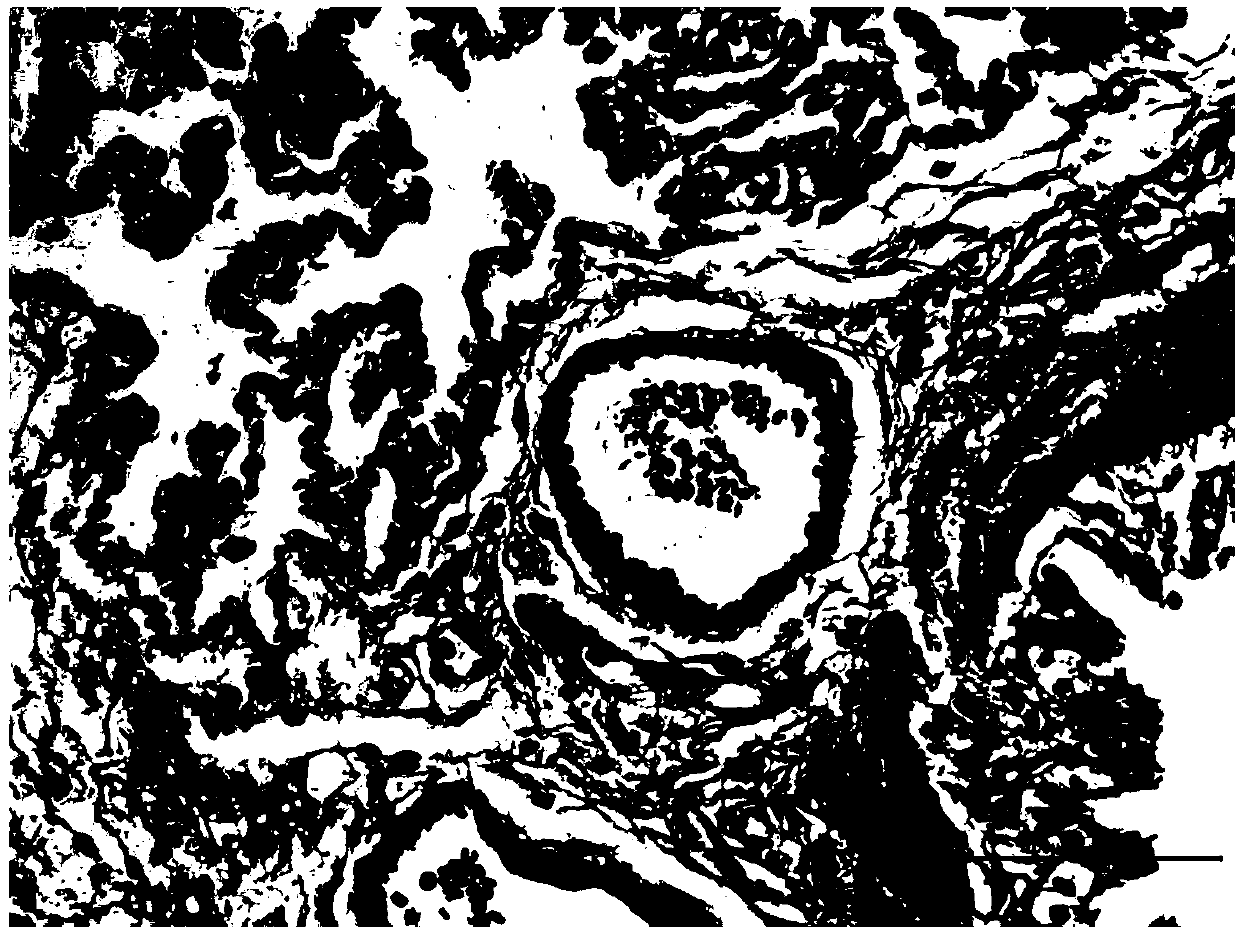
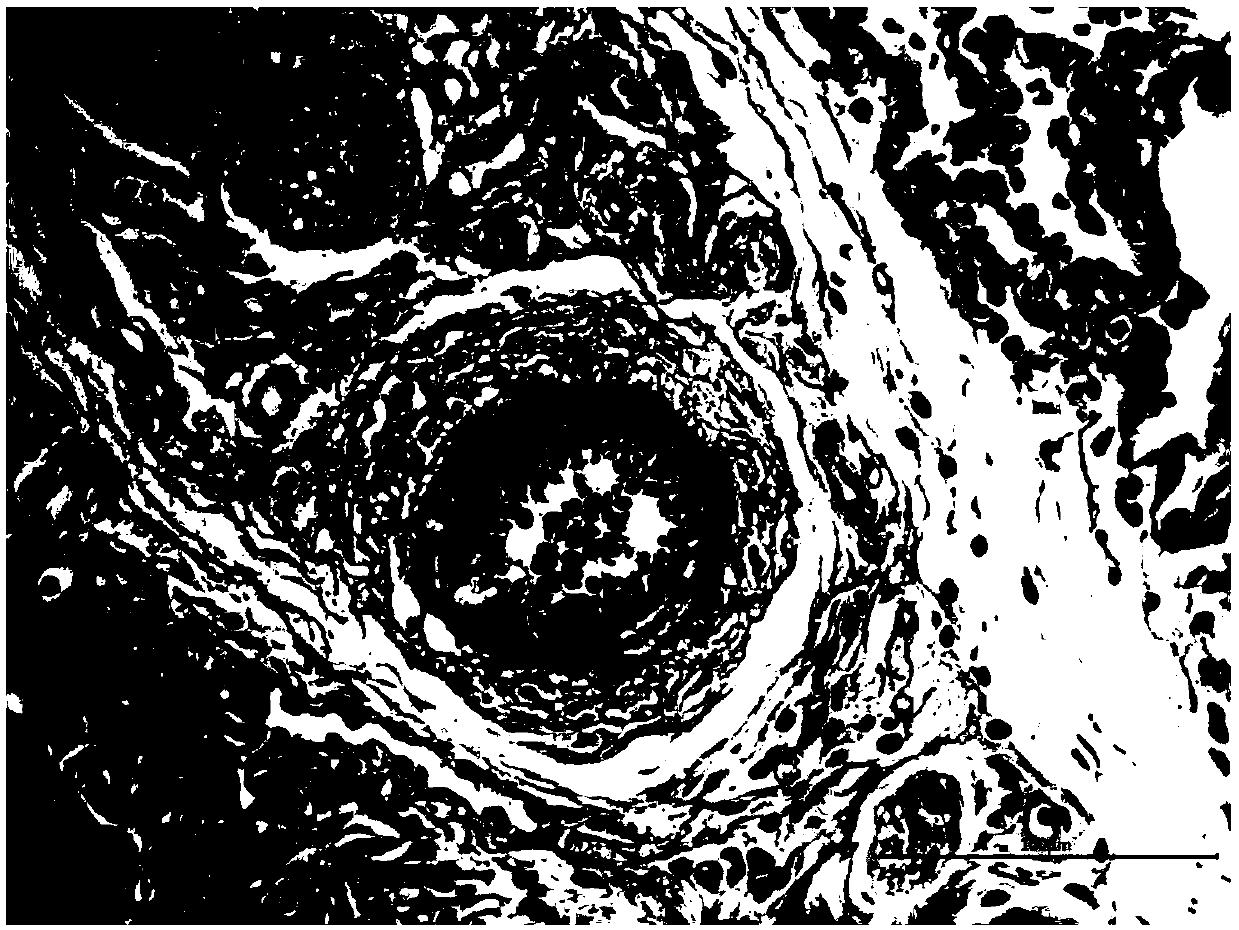
Application of ibrutinib in preparation of drugs for treating chronic hypoxic pulmonary hypertension
A technology of pulmonary arterial hypertension and ibrutinib, applied in drug combinations, pharmaceutical formulas, organic active ingredients, etc., can solve the problems of irreversible pulmonary hypertension process, uncertain effect of targeted drug therapy, etc., and achieve the effect of drug safety
- Summary
- Abstract
- Description
- Claims
- Application Information
AI Technical Summary
Problems solved by technology
Method used
Image
Examples
Embodiment 1
[0019] Ibrutinib is added with pharmaceutically acceptable excipients and prepared into various dosage forms according to conventional methods, such as various specifications of liquid injections, powder injections, emulsions for injection, tablets, pills, capsules, ointments, creams, patches , Liniments, powders, sprays, implants, drops, suppositories, ointments, confectionery, etc.
[0020] The route of administration of Ibrutinib includes various routes of administration: oral administration, injection administration, implant administration, intracavity administration, sublingual administration, anal administration, transdermal administration, internal and external application, etc. .
experiment example 1
[0022] In order to prove the technical means, purpose and experimental effect of the experimental invention, the following will further illustrate the present invention with specific experimental examples.
[0023] (1) Experimental materials
[0024] 1. Laboratory animals
[0025] SD rat, male, 350±10g, purchased from Beijing Weitonglihua Laboratory Animal Technology Co., Ltd. The experimental animals were placed in the modeling area for 7 days after they were purchased.
[0026] Animal feeding conditions: constant temperature 25±1℃, constant humidity 55±5%, keep light and dark for 12 hours each, feed and water normally.
[0027] 2. Experimental drugs and configuration methods:
[0028] Ibrutinib (ibrutinib), purchased from Selleck, storage conditions: 4°C, protected from light. Ibrutinib 22.5 mg was accurately weighed, stirred until it was completely dissolved in 25 μl DMSO, diluted with 2.5 ml of saline, and stored.
[0029] (2) Experimental method
[0030] 1. Model construction and dr...
PUM
 Login to View More
Login to View More Abstract
Description
Claims
Application Information
 Login to View More
Login to View More - R&D
- Intellectual Property
- Life Sciences
- Materials
- Tech Scout
- Unparalleled Data Quality
- Higher Quality Content
- 60% Fewer Hallucinations
Browse by: Latest US Patents, China's latest patents, Technical Efficacy Thesaurus, Application Domain, Technology Topic, Popular Technical Reports.
© 2025 PatSnap. All rights reserved.Legal|Privacy policy|Modern Slavery Act Transparency Statement|Sitemap|About US| Contact US: help@patsnap.com